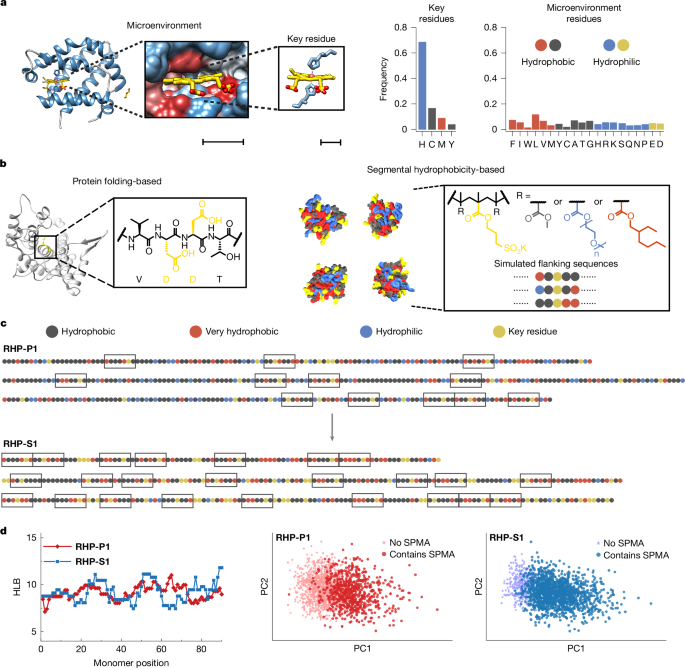
Scientists Uncover Molecular Secrets by Making Light Disappear in Liquids
Researchers have used high-harmonic spectroscopy to observe ultrafast electron dynamics in liquids, revealing how specific molecular interactions, like hydrogen bonding, can disrupt electron motion and suppress light emission, with potential implications for chemistry and biology.