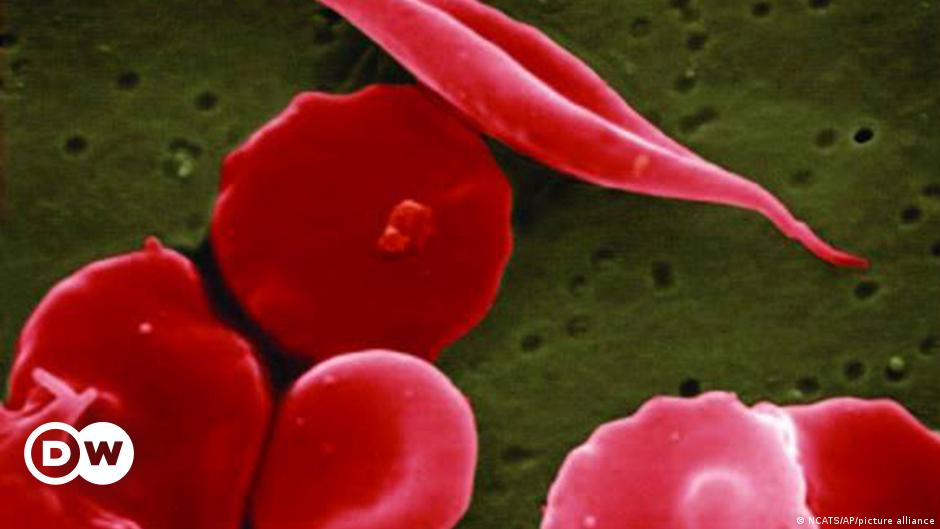
FDA Approves CRISPR Gene-Editing for Sickle Cell Disease Treatment
The US Food and Drug Administration (FDA) has approved two groundbreaking gene-editing treatments, Casgevy and Lyfgenia, for sickle cell disease. Casgevy, based on the CRISPR gene editing tool, targets the problematic gene in a patient's bone marrow stem cells, resulting in the production of properly functioning hemoglobin. Lyfgenia utilizes a harmless virus to insert a gene into patients' stem cells. Sickle cell disease is a rare and life-threatening blood disorder that primarily affects people of African or Caribbean descent. The approval of these treatments offers hope for individuals with sickle cell disease, who previously had limited treatment options.

