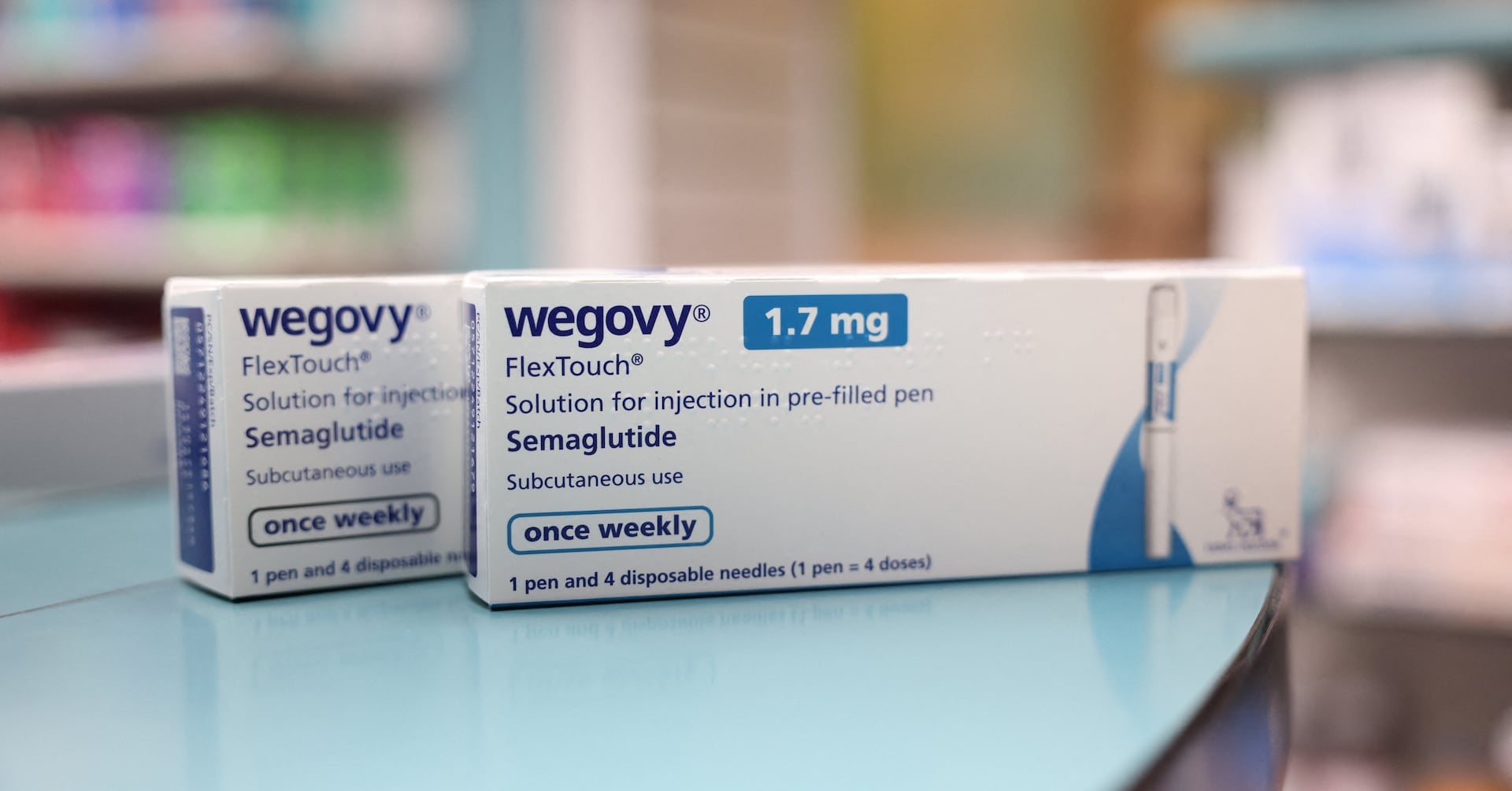
"Wegovy: Medicare Expands Coverage for Heart-Related Benefits"
Novo Nordisk and Eli Lilly are inching closer to justifying their high valuations as the U.S. government announces plans to cover weight loss drug Wegovy for Medicare patients, potentially including other drugs like Zepbound for cardiovascular disease. This move could make the pharma giants' valuations seem less far-fetched, especially as obesity treatments become bigger sellers with Medicare's support.