Unveiling the Genetic Basis of DNA Damage and Micronucleus Formation
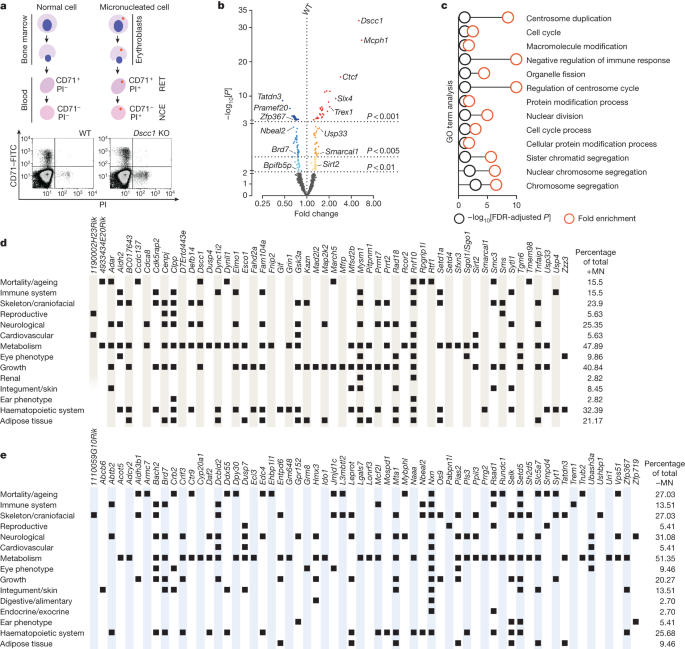
A study identified genetic factors that regulate micronucleus (MN) formation in vivo by screening over 6,000 mice and found genes that either increased or decreased MN formation. The study also integrated findings with a genome-wide association study and identified potential human disease relevance. DSCC1, a gene involved in sister chromatid cohesion, was found to be critical for genome maintenance, and its deficiency led to phenotypes associated with genomic instability. Additionally, a genome-wide CRISPR–Cas9 screen revealed that SIRT1 inhibition could rescue the proliferation defect of DSCC1-deficient human cells, suggesting a potential therapeutic target for further investigation.