Mapping Tumor Immune Niches in Diffuse Large B Cell Lymphoma
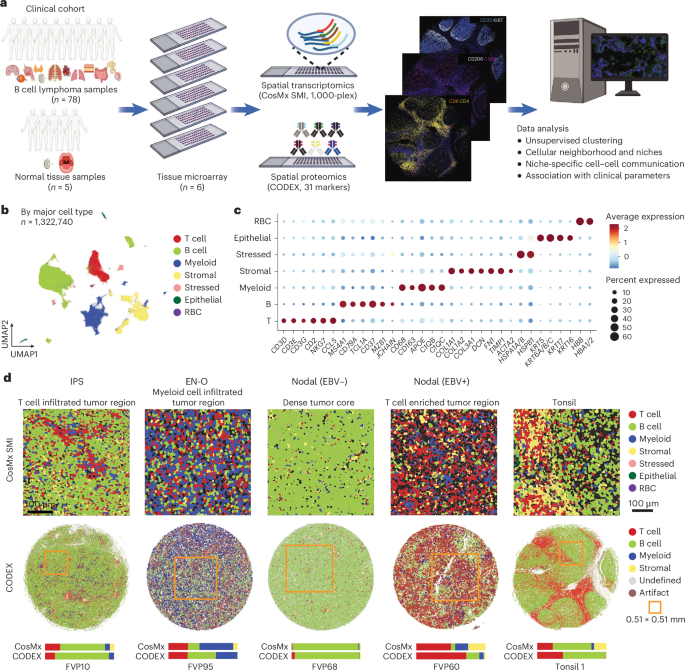
This study used spatial transcriptomics, proteomics, and genomics to analyze the tumor immune microenvironments in diffuse large B-cell lymphoma (DLBCL), identifying distinct cellular neighborhoods and communication patterns that influence immune cell function and tumor behavior, with implications for targeted immunotherapy.


