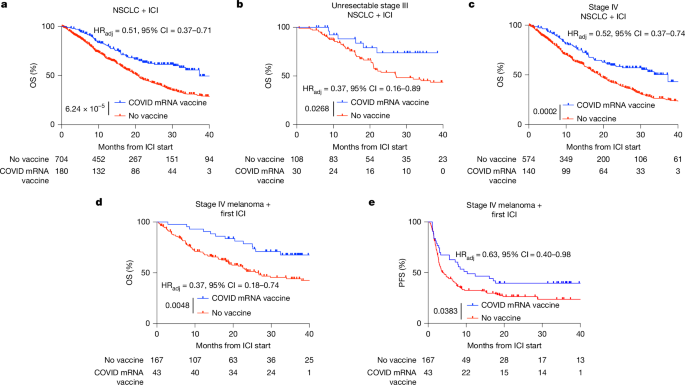
Update on COVID-19 Vaccine Antigen Composition
The WHO's TAG-CO-VAC recommends updating COVID-19 vaccine antigens to monovalent LP.8.1 to better target circulating SARS-CoV-2 variants, while noting that existing JN.1 lineage vaccines remain suitable. Ongoing surveillance and data collection are emphasized to adapt vaccine strategies as the virus evolves.