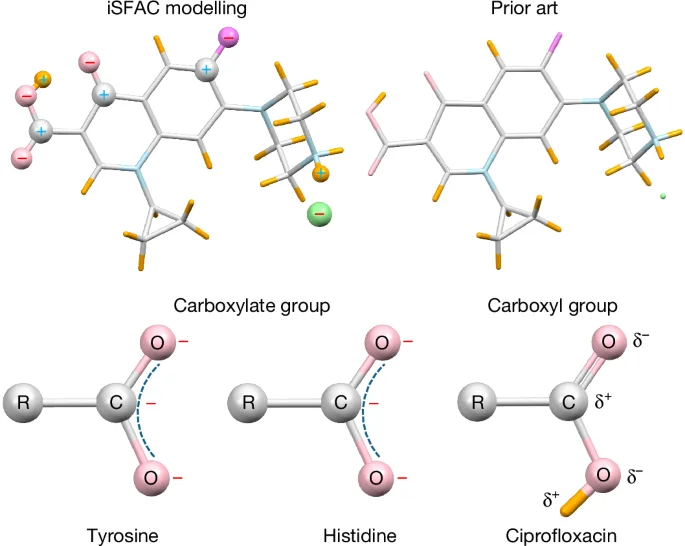
Electron Diffraction Reveals Partial Charges
The article introduces iSFAC modelling, an innovative experimental method using 3D electron diffraction to determine absolute partial charges of atoms in molecules, applicable across various chemical classes. It enhances understanding of chemical bonds by capturing the influence of the chemical environment, aligns well with quantum-mechanical calculations, and offers insights into molecular interactions and properties, even in inorganic structures like zeolites.