"Unveiling the Hidden Power of Van der Waals Crust in Describing Chemical Bonds"
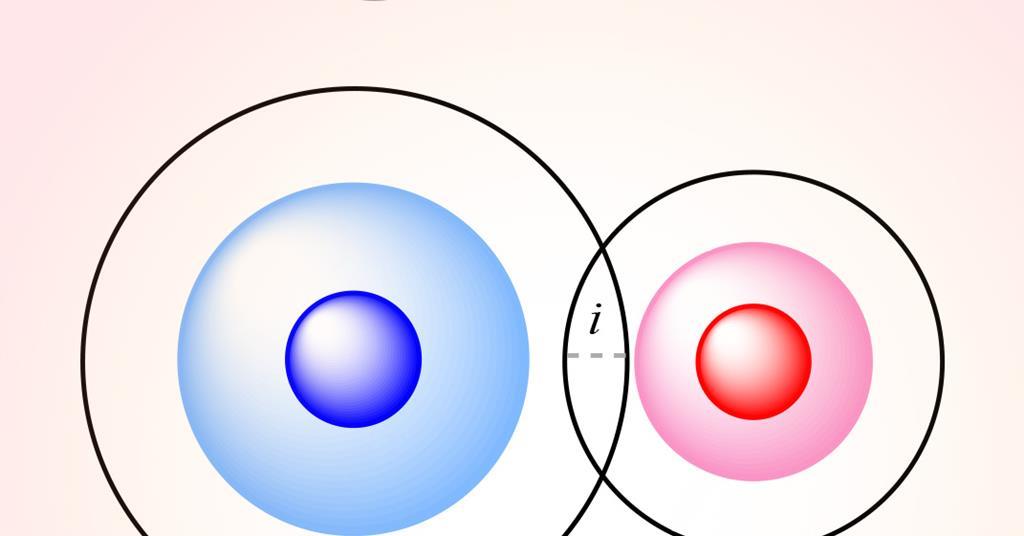
Researchers in Spain have developed a new parameter called the penetration index, which describes the degree of interpenetration of the van der Waals crusts of two atoms. This parameter can replace previous distance measurements and be applied to a wide range of chemical bonds. The penetration index provides a quantitative measure of the degree of interaction between atoms, independent of their size. It has been successfully applied to hydrogen bonding and other bonding interactions, allowing for more precise descriptions and a better understanding of chemical bonding and structure. However, the method has some limitations when applied to ionic crystals due to its reliance on covalent radii rather than ionic radii.
Reading Insights
0
2
3 min
vs 4 min read
85%
737 → 110 words
Want the full story? Read the original article
Read on Chemistry World