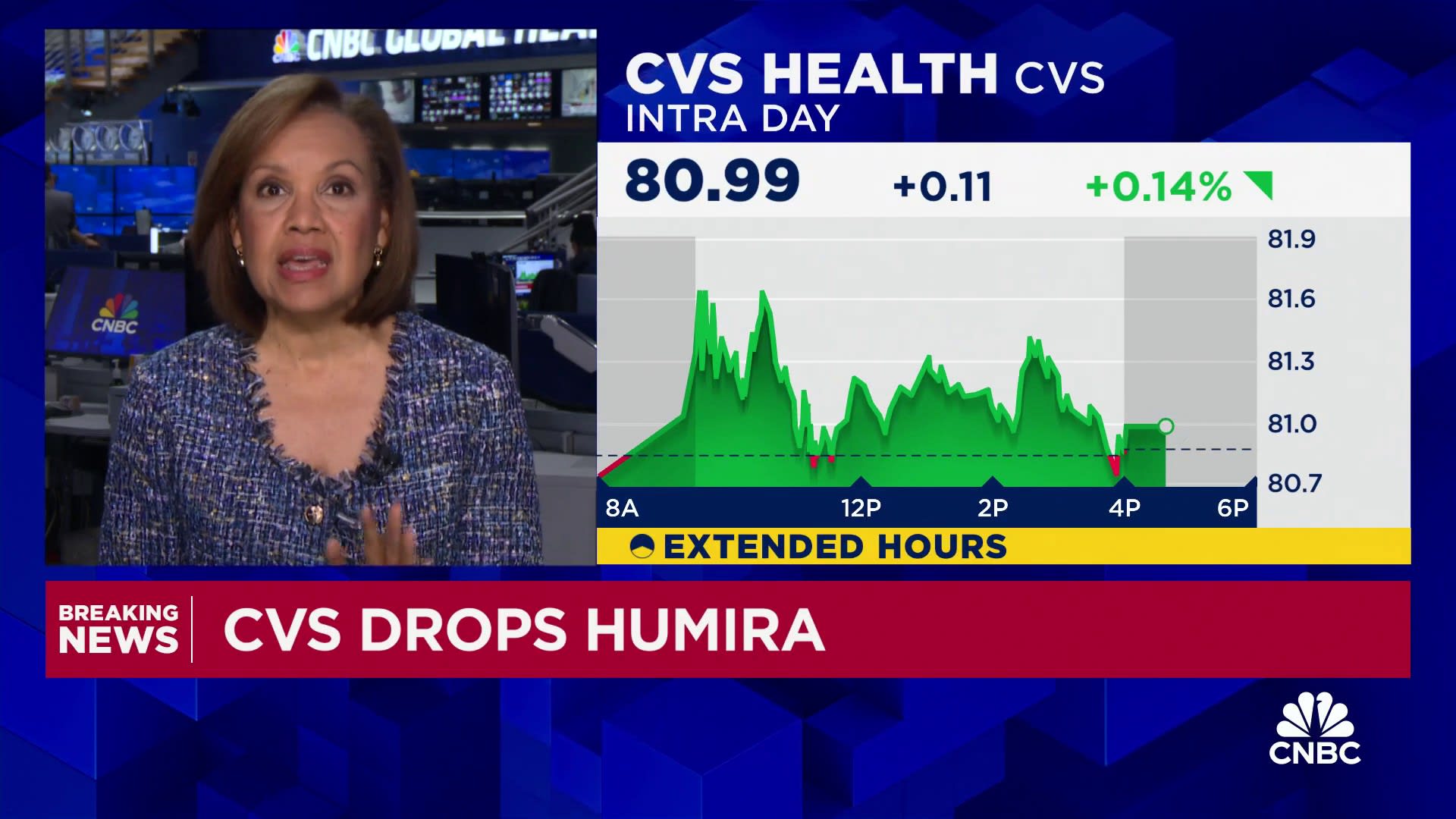
FDA Accelerates Generic Biologic Approvals to Reduce Drug Costs
The FDA announced plans to accelerate the approval process for generic biologic medicines, or biosimilars, to increase competition and reduce high drug prices in the U.S., which are significantly higher than in other countries. The reforms aim to cut the approval timeline in half, simplify studies, and remove barriers to market entry, potentially saving billions in healthcare costs and providing more affordable options for patients.