Immunization in the Crosswinds: Courts, States, and Agencies Reshape U.S. Vaccine Policy
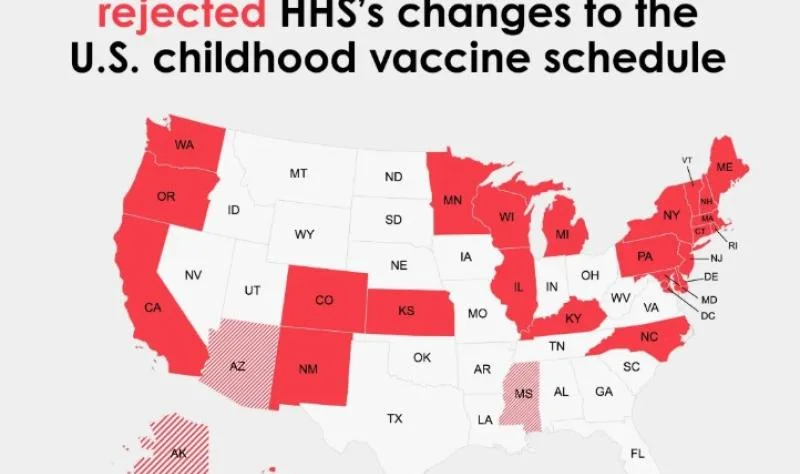
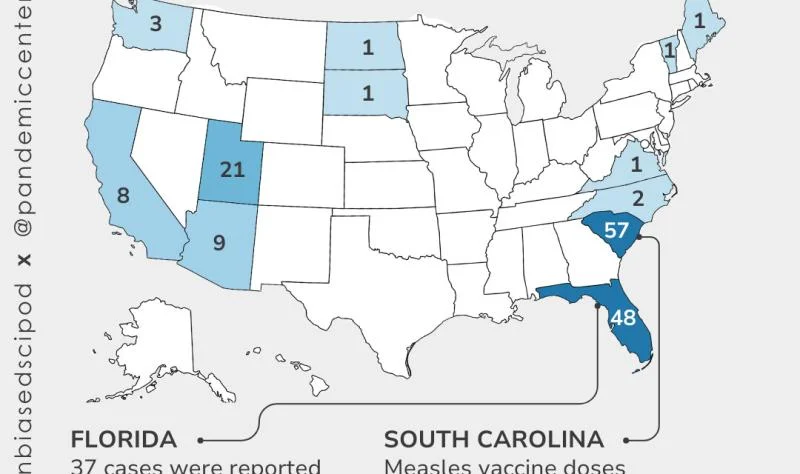
This biweekly briefing maps sweeping shifts in U.S. vaccine policy: federal court battles over pediatric vaccine scheduling and ACIP governance, state efforts to roll back school vaccine requirements, regulatory reversals on Moderna’s mRNA flu vaccine, and a leadership shake-up at the CDC, all set against widening data gaps and new data tools from public health researchers and advocates.