"GLP-1 Agonists Linked to Increased Risk of Stomach Paralysis in Weight Loss"
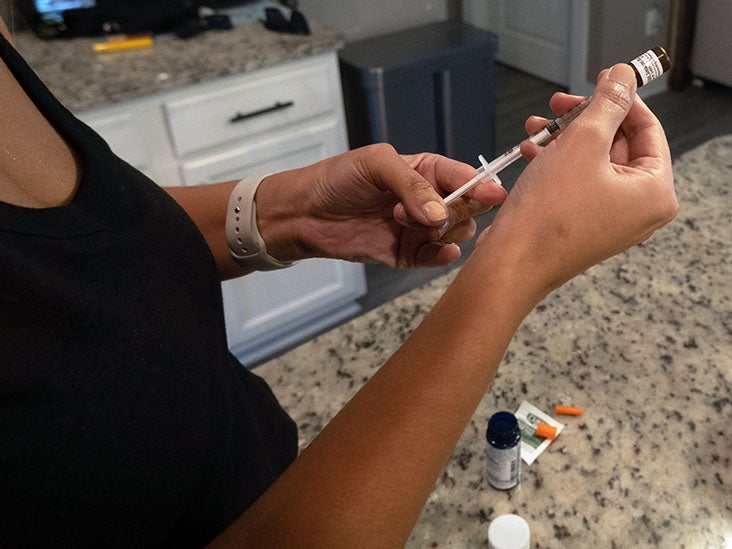
New research from the University of British Columbia has found a link between the use of GLP-1 agonists, such as Ozempic and Wegovy, and an increased risk of stomach paralysis, pancreatitis, and bowel obstruction. GLP-1 agonists are a class of drugs commonly used for weight loss. Previous studies have also shown potential side effects of these medications, including nausea, vomiting, diarrhea, and headache. The recent research analyzed health insurance claim records of over 16 million people in the United States and found that those who took GLP-1 agonists had a significantly higher risk of developing gastrointestinal conditions compared to those who took a different weight loss medication. Experts emphasize the importance of informed patient consent and further research to fully understand the risks associated with these medications.