"Uncovering the Role of Human Cell Proteins in Accelerating Aging"
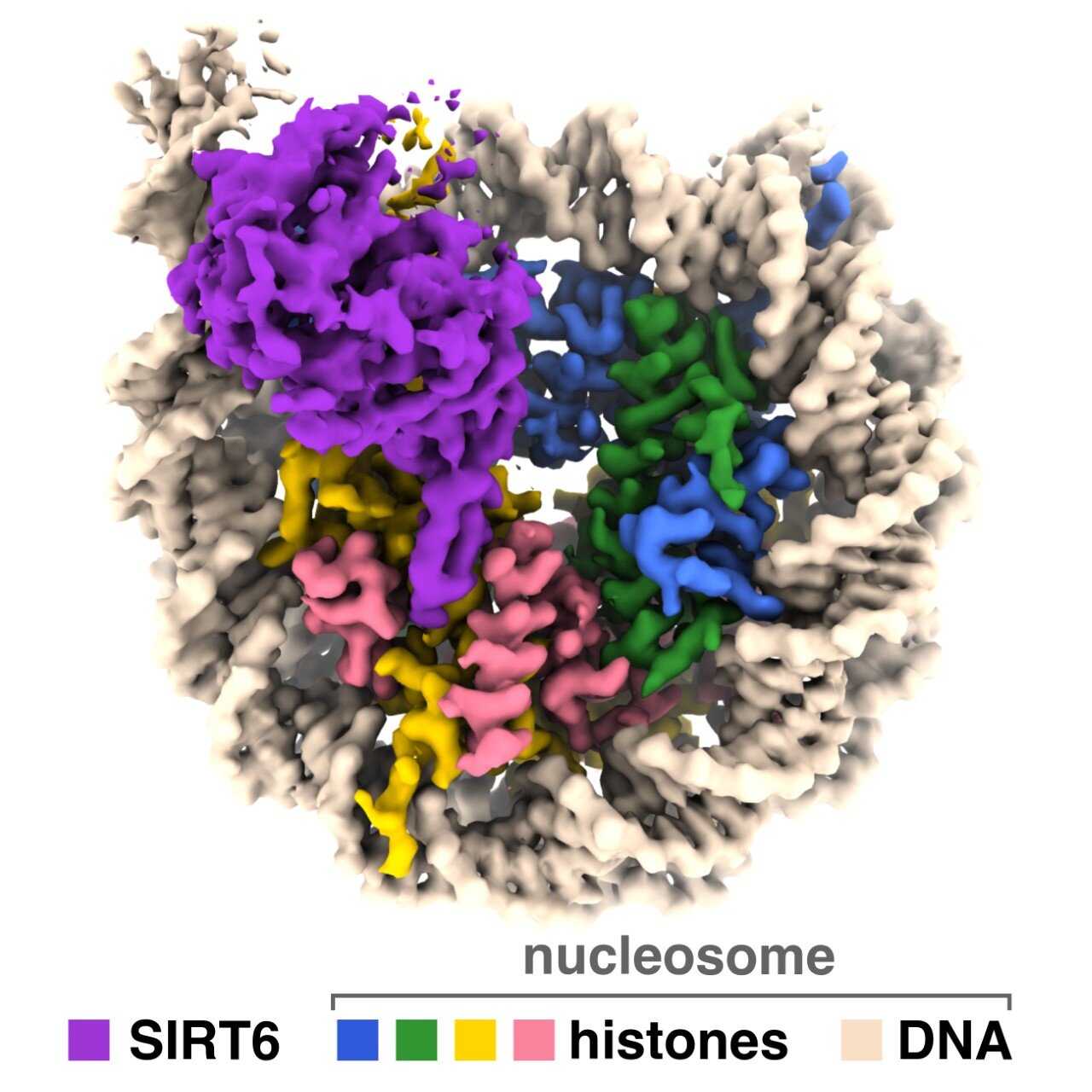
Scientists have found that an increased level of the protein PAPPA, driven by a family of signaling proteins called sirtuins, accelerates aging in various human cells. This discovery was made by researchers from the Chinese Academy of Sciences, shedding light on the mechanisms behind aging in humans.