"Serum Institute of India Develops New Vaccines for Malaria and Dengue"
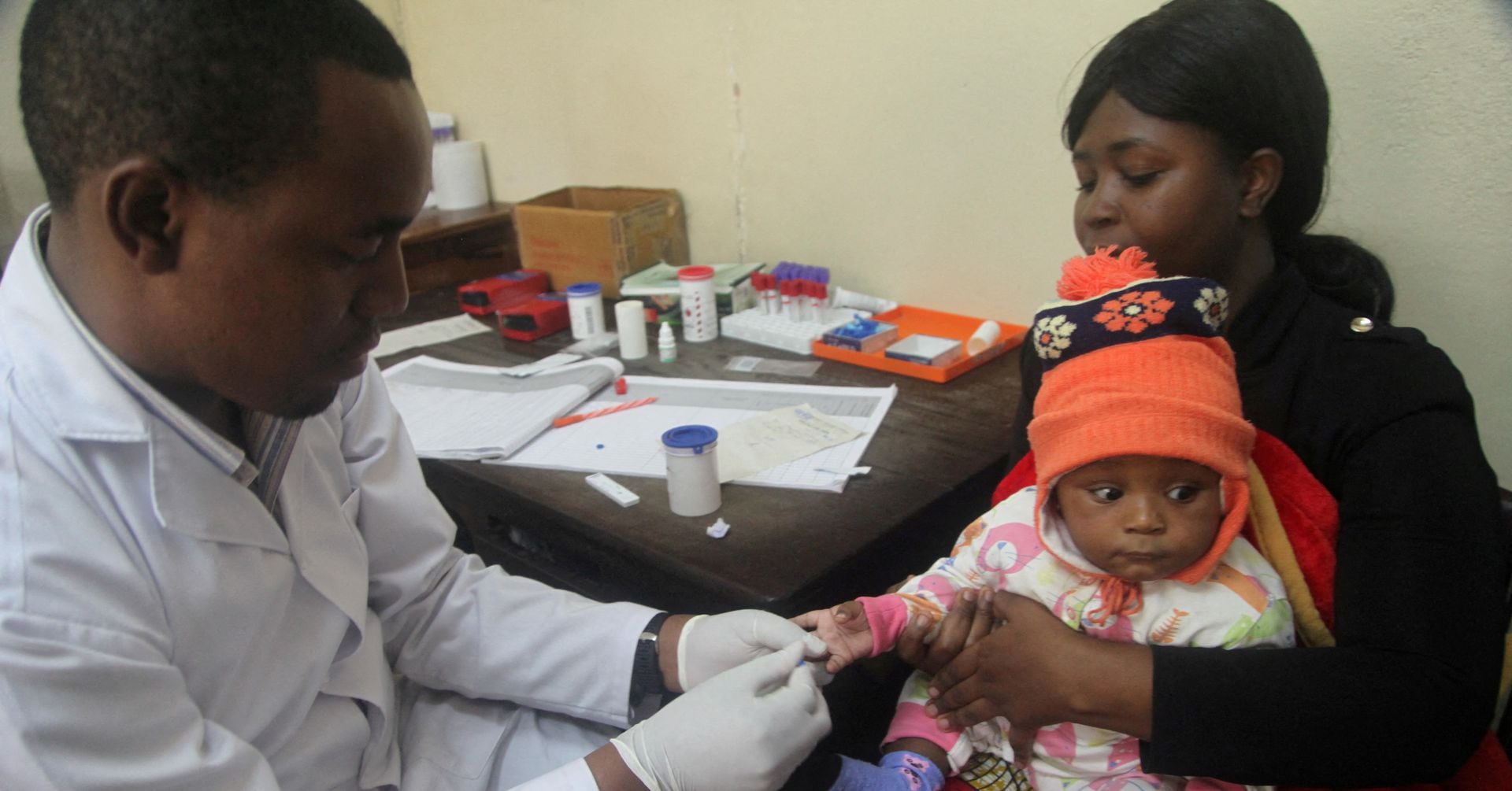
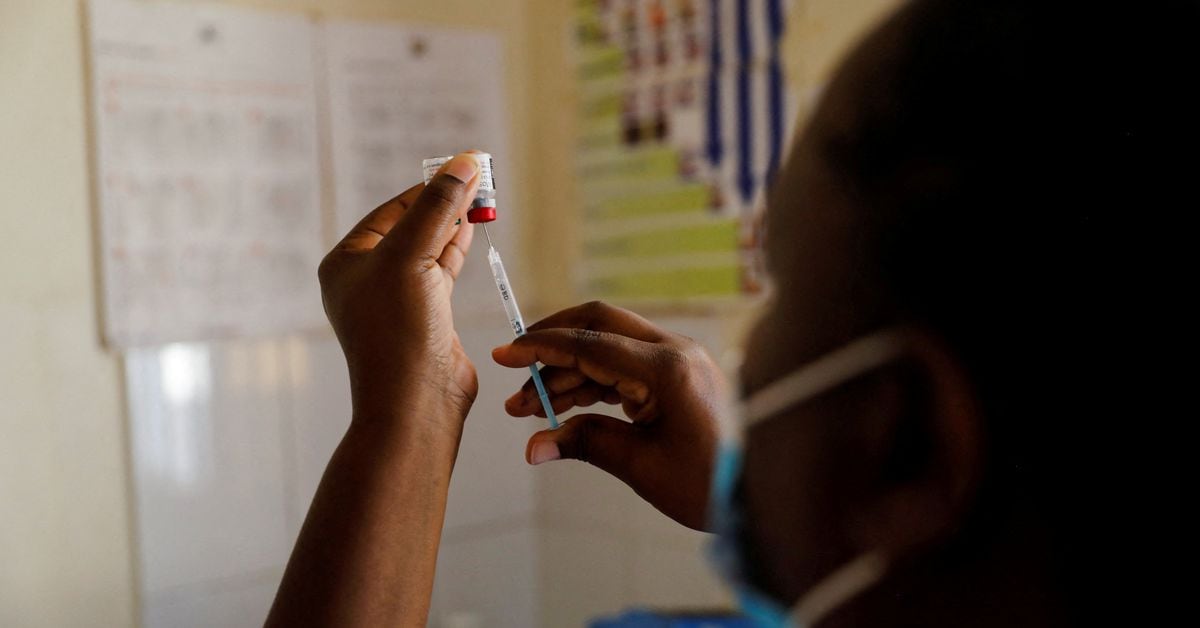
Serum Institute of India, known for producing COVID-19 vaccines, is repurposing its facilities to manufacture vaccines for diseases like malaria and dengue. The company aims to boost total production by two and a half billion doses and has already produced 25 million doses of a malaria vaccine. Additionally, it is testing a single-dose vaccine for dengue and plans to focus on exporting these vaccines to other countries. Serum Institute of India is also in talks with governments to utilize its facilities in the event of future outbreaks and estimates a total production capacity of as much as 4 billion doses.