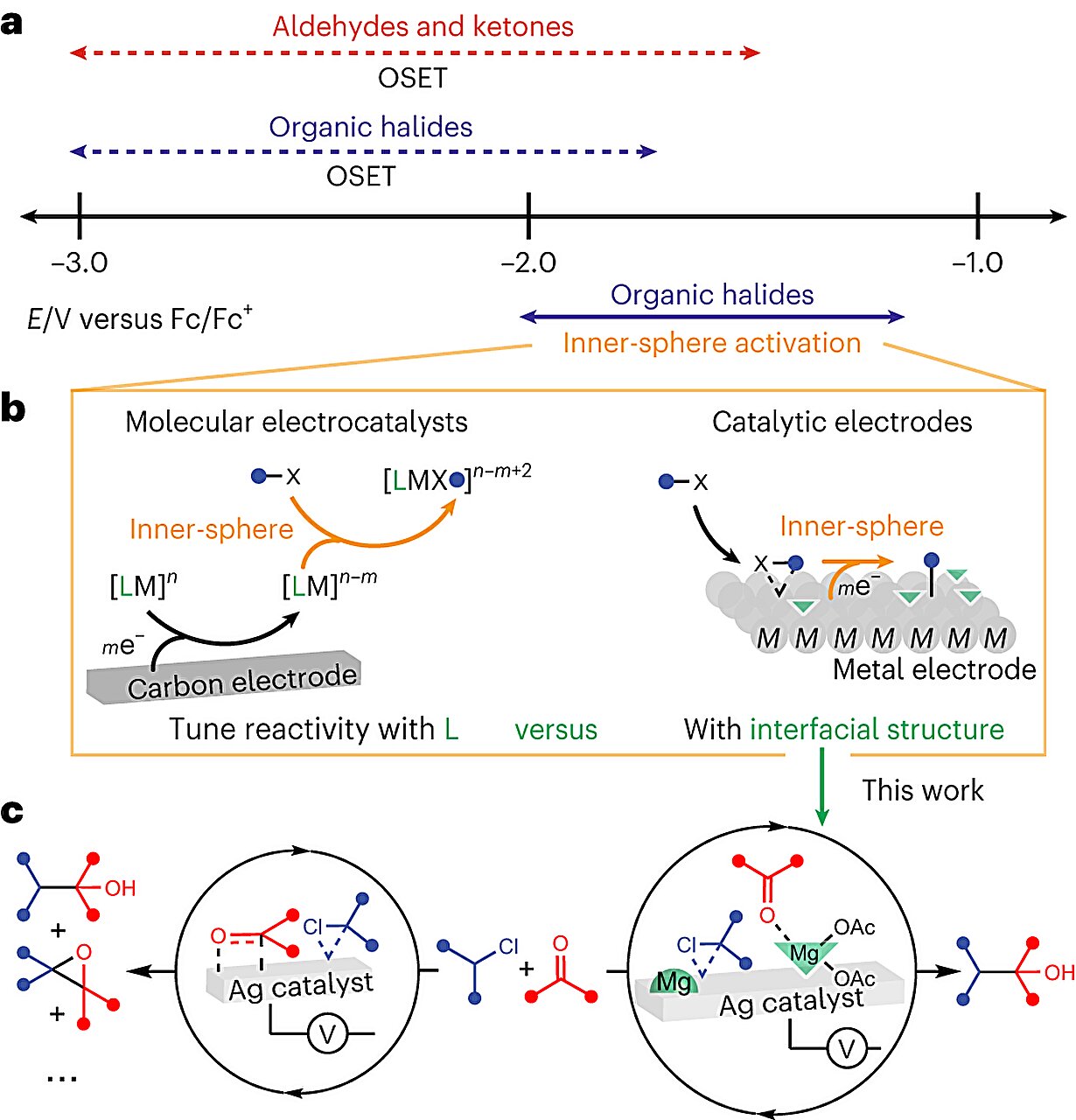
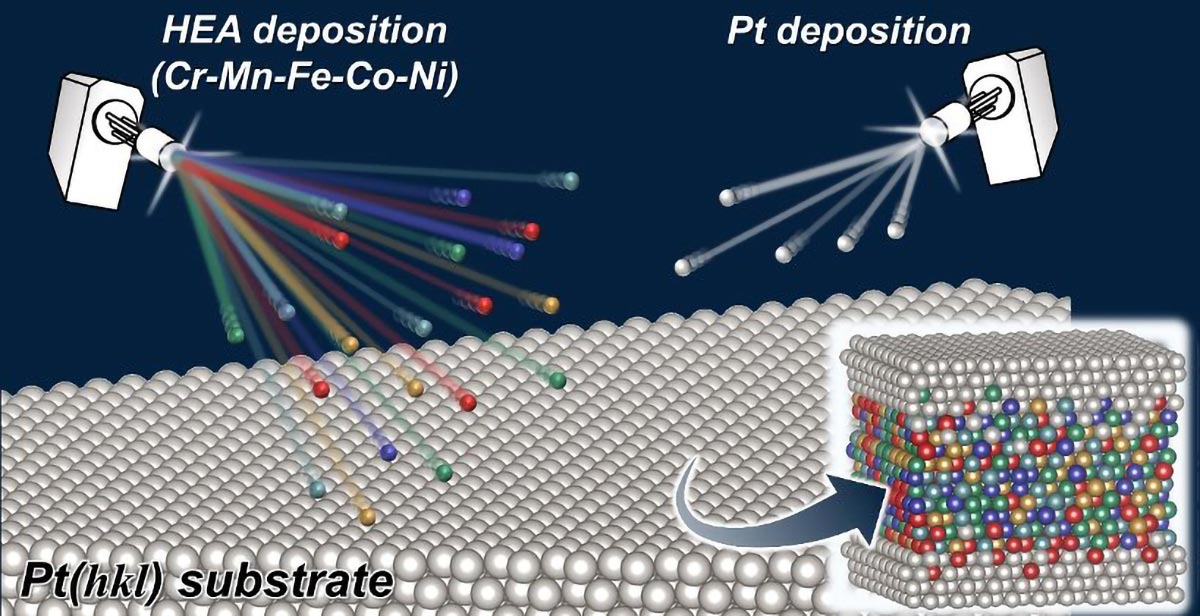
Three-Layer Electrode Bridges CO2 Capture and Conversion to Formic Acid
A three-layer gas-diffusion electrode can simultaneously capture CO2 from real-world gas streams (including dilute ambient air and simulated flue gas) and convert it directly into formic acid, outperforming existing electrodes under realistic conditions. The device combines a CO2-selective capture layer, a gas-permeable carbon paper, and a tin(IV) oxide catalyst, offering a simpler, on-site route to carbon utilization with potential adaptation to other greenhouse gases in the future.