"Revolutionary Gene Therapy Eyedrops Offer Hope for Restoring Vision to Millions"
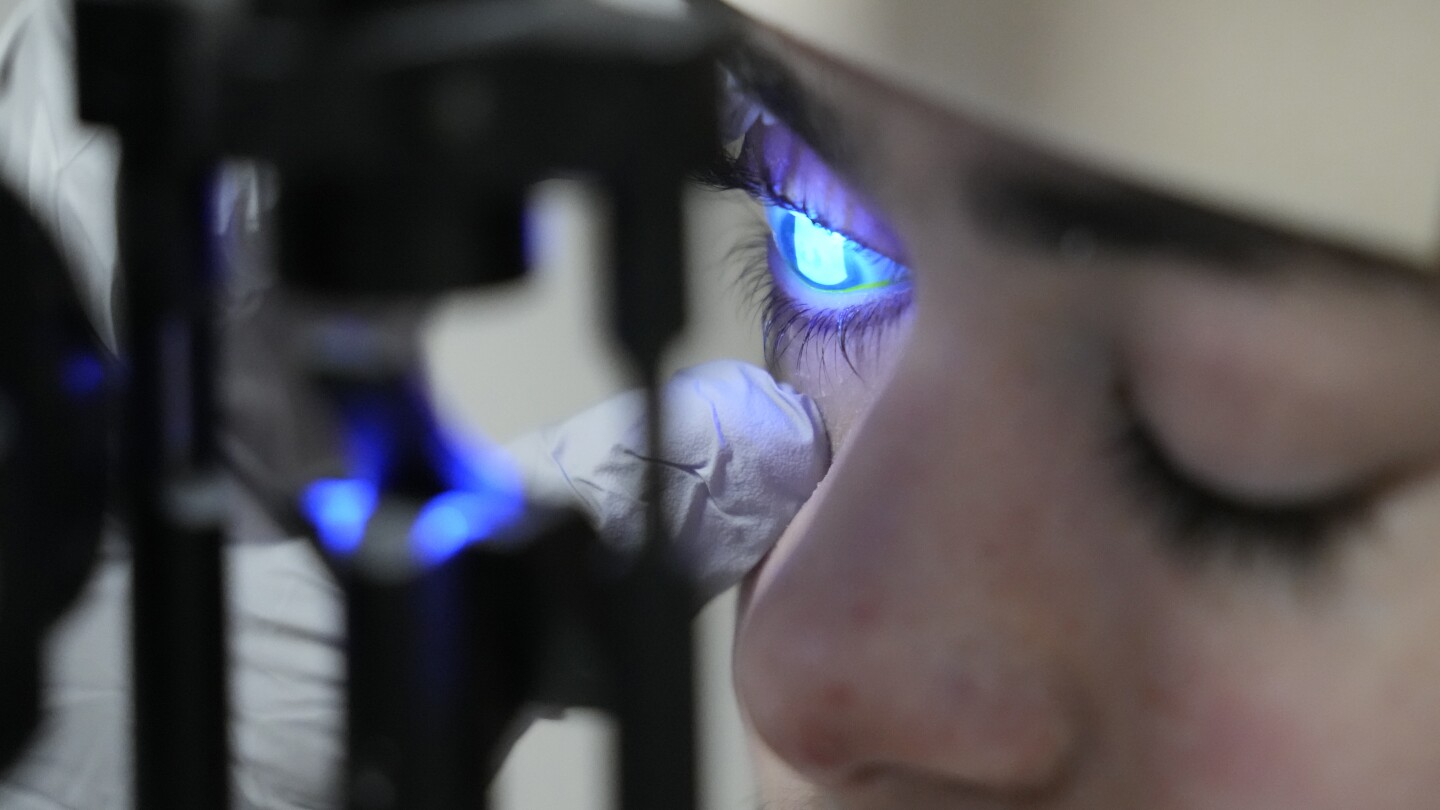
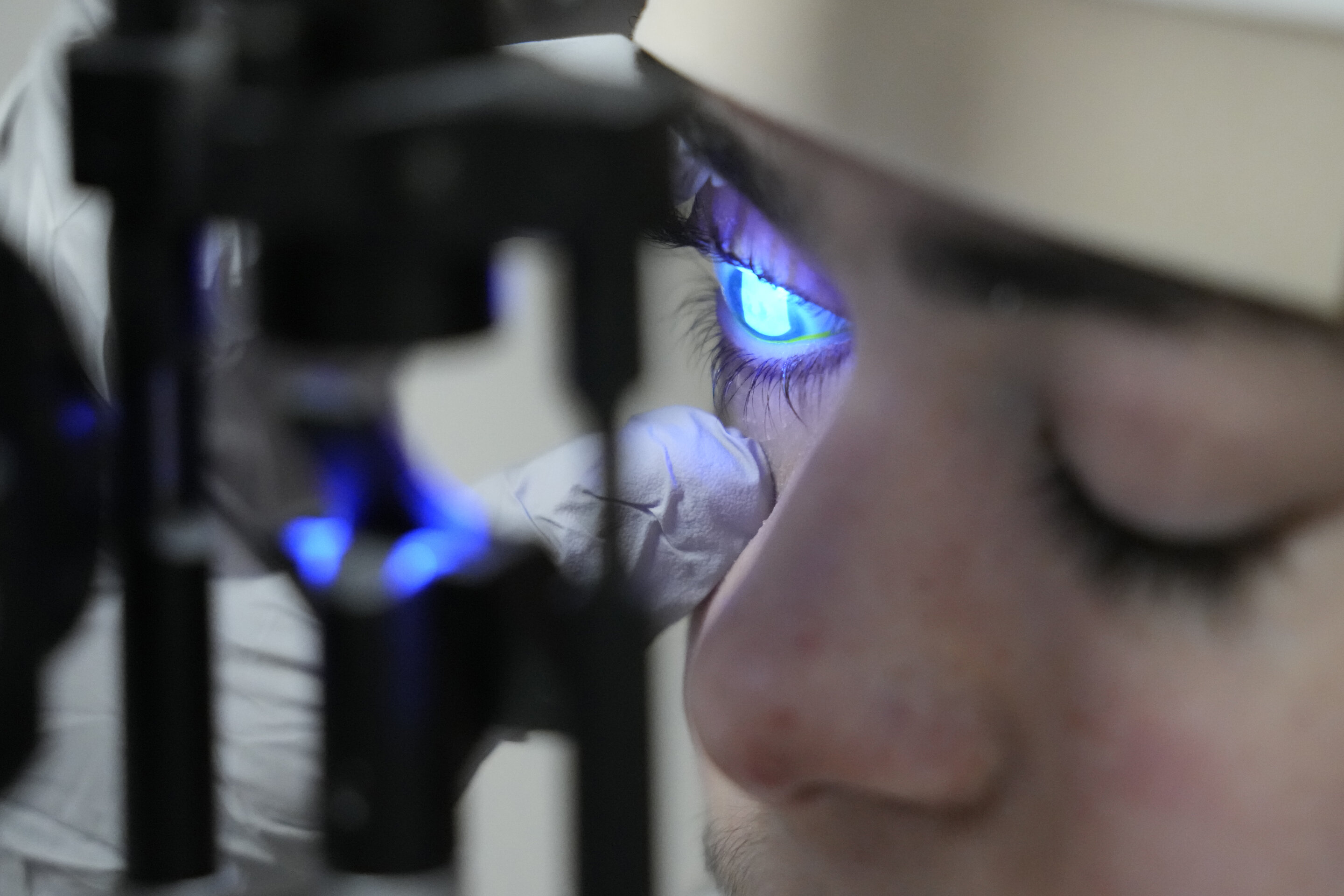
Gene therapy eyedrops have successfully restored the sight of a 14-year-old boy with dystrophic epidermolysis bullosa, a rare genetic condition that causes blisters in the eyes. The treatment, called Vyjuvek, uses an inactivated herpes simplex virus to deliver working copies of the gene responsible for producing collagen 7, which holds together the skin and corneas. After two years of testing and approvals, the boy's vision significantly improved, and similar therapies could potentially help millions of people with other eye diseases. The gene therapy eyedrops have the potential to be adapted for various conditions, offering hope for future patients.