13-Year-Old's Chest Pain Reveals Rare Near-Death Heart Condition
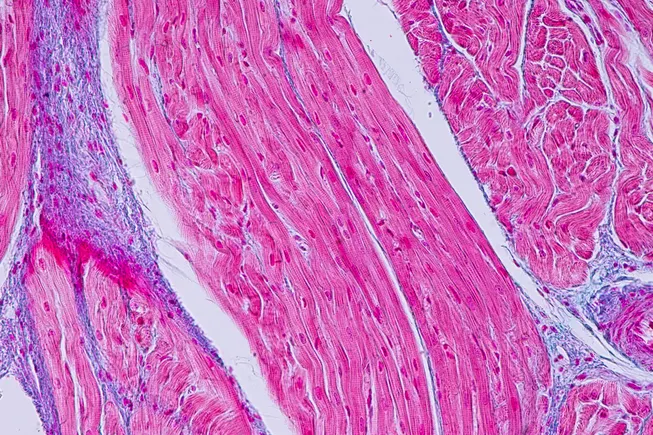
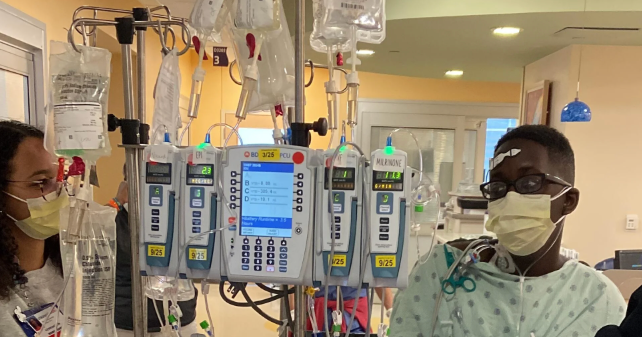
A 13-year-old boy with a rare genetic condition called Danon disease, which affects only about 300 families worldwide, was diagnosed with severe heart failure and required a heart transplant. His mother, who also has the disease, and he are among the few documented African-American cases. The story highlights the importance of support systems for young transplant patients, leading to the creation of a virtual community called Transplant Teenz to help teenagers cope with their condition.