New Pill Baxdrostat Shows Promise in Treating Resistant Hypertension and Protecting Kidneys
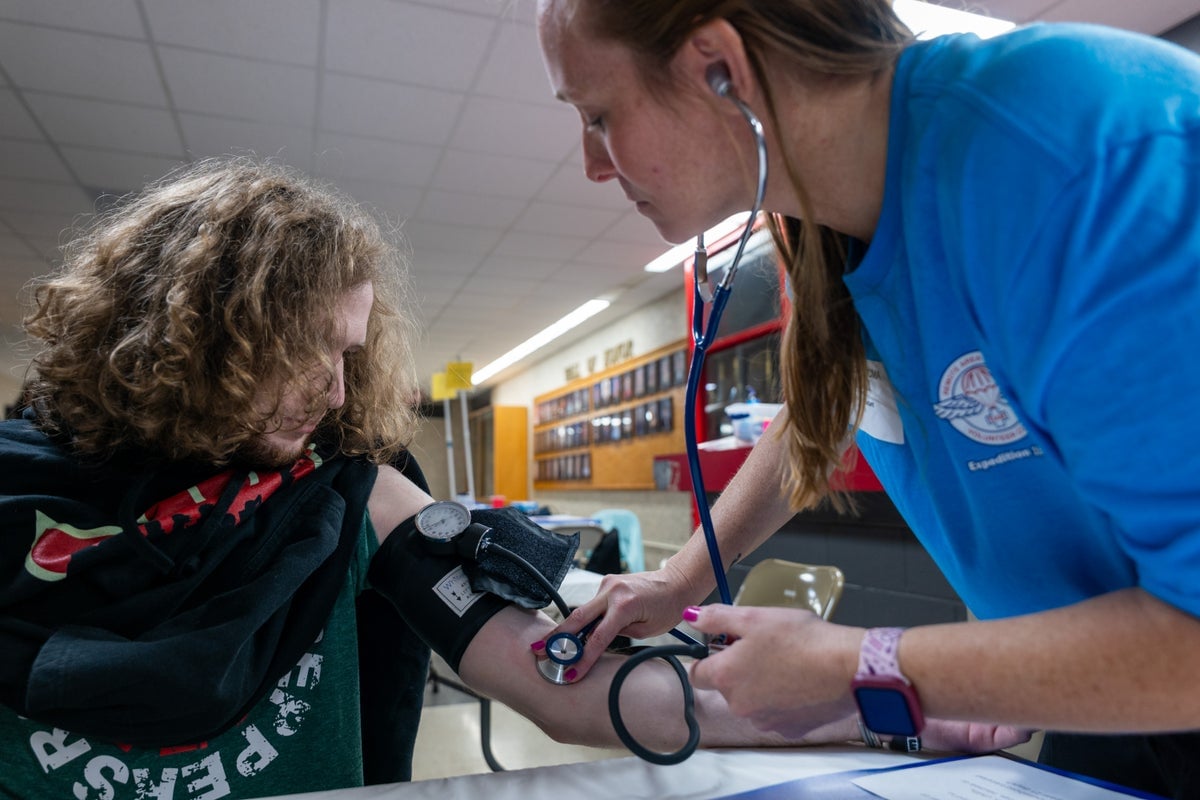
A new drug called baxdrostat shows promise in lowering blood pressure and reducing kidney damage markers in patients with chronic kidney disease and uncontrolled hypertension, with ongoing Phase 3 trials to assess its long-term effects.