FDA Approves First Chikungunya Virus Vaccine for Adults 18+ in the US
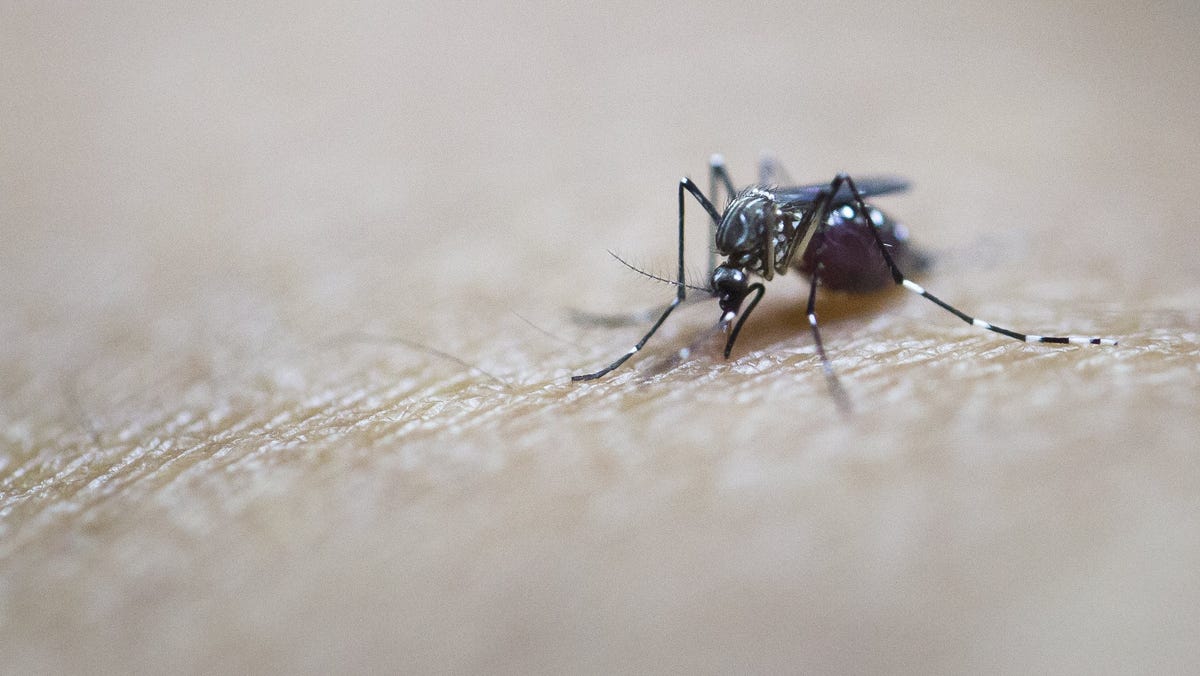
The FDA has granted approval for the first vaccine against the chikungunya virus, a disease primarily transmitted through mosquito bites. The vaccine, Ixchiq, is intended for individuals aged 18 and older who are at a higher risk of exposure to the virus. Chikungunya virus poses a global health threat, with millions of cases reported in the past 15 years. While the highest risk is in tropical and subtropical regions, the virus has spread to other areas. Symptoms include fever, joint pain, rash, and headache. The vaccine, which contains a weakened version of the virus, was evaluated in clinical studies and showed common side effects such as headache and muscle pain. Researchers will conduct a postmarket study to assess potential risks.
Reading Insights
0
1
2 min
vs 3 min read
77%
530 → 120 words
Want the full story? Read the original article
Read on USA TODAY