"Philips Halts U.S. CPAP Sales Amid Recall Crisis and FDA Deal"
TL;DR Summary
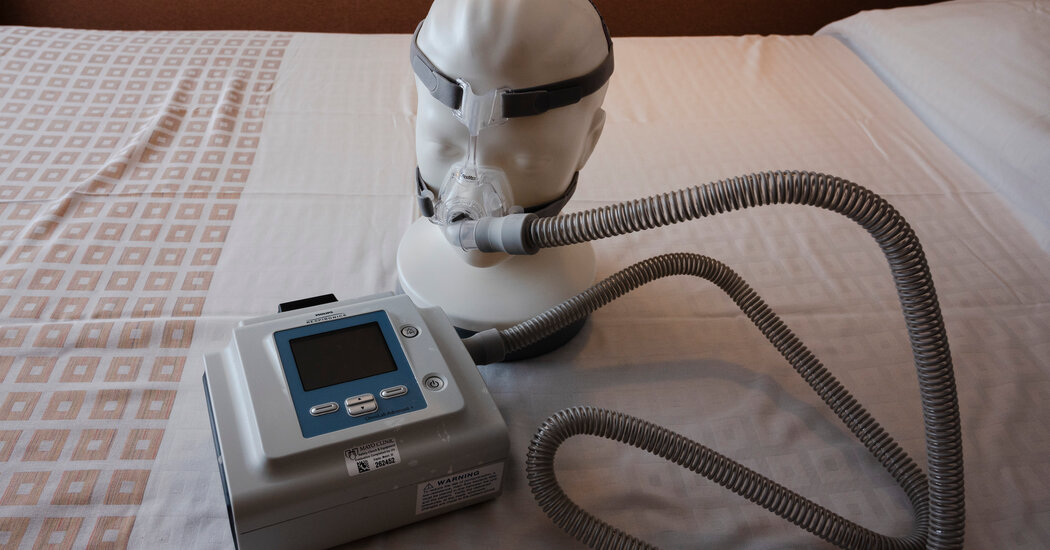
Philips Respironics has suspended sales of all its breathing machines in the United States following a settlement with the FDA over ongoing issues with the devices, including reports of foam and potentially toxic gases being released into consumers' airways. The company must meet a list of standards in a "multiyear" plan before it can resume business in the U.S. and will continue to repair existing devices. The FDA has pushed back on some of the company's updated claims and the agreement is pending finalization and filing with the court.
- Philips Suspends Sales of CPAP and Other Breathing Devices After Recall The New York Times
- Philips' U.S. sales of sleep apnea devices face years-long halt after FDA deal CNBC
- Philips announces its 2023 Fourth-Quarter and Annual Results Philips
- Amid recall crisis, Philips agrees to stop selling troubled sleep apnea machines in the United States Pittsburgh Post-Gazette
- Philips to halt CPAP sales in the U.S. as it takes $393 million provision MarketWatch
Reading Insights
Total Reads
0
Unique Readers
7
Time Saved
2 min
vs 3 min read
Condensed
78%
402 → 89 words
Want the full story? Read the original article
Read on The New York Times