"Philips Halts U.S. CPAP Sales Amid Recall Crisis and FDA Deal"
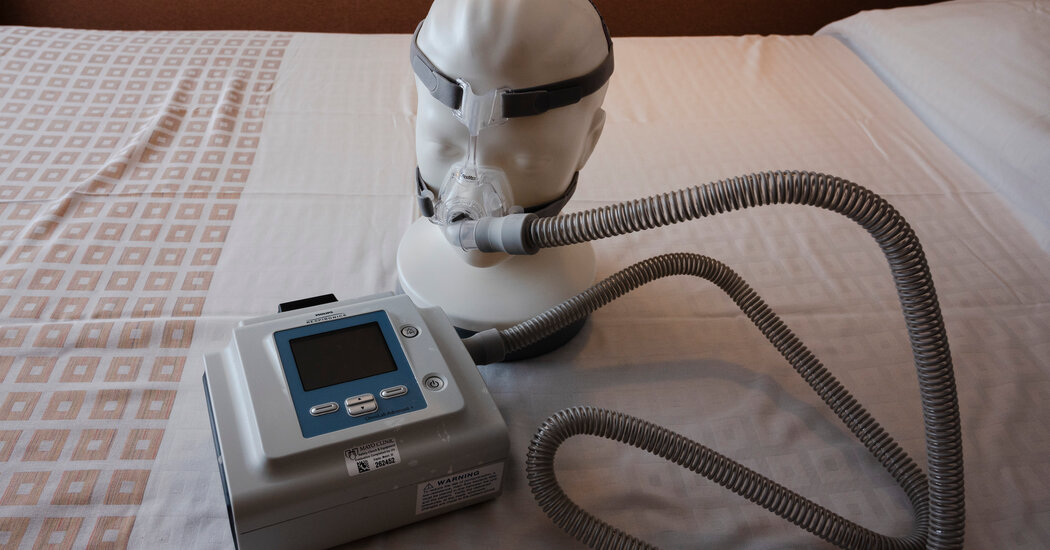
Philips Respironics has suspended sales of all its breathing machines in the United States following a settlement with the FDA over ongoing issues with the devices, including reports of foam and potentially toxic gases being released into consumers' airways. The company must meet a list of standards in a "multiyear" plan before it can resume business in the U.S. and will continue to repair existing devices. The FDA has pushed back on some of the company's updated claims and the agreement is pending finalization and filing with the court.

