FDA Investigates Potential Cancer Risk of Cutting-Edge CAR-T Therapy
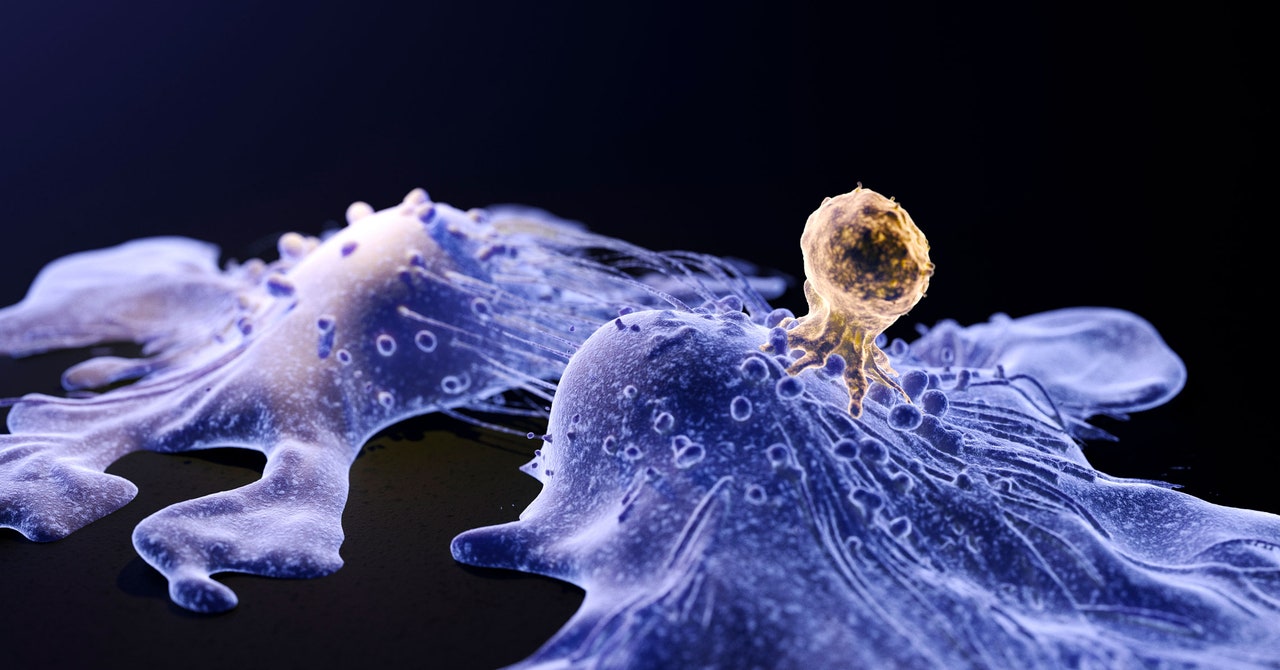
The US Food and Drug Administration (FDA) is investigating the potential risk of a cutting-edge cancer treatment, CAR-T cell therapy, causing secondary malignancies. The therapy uses harmless viruses to deliver new genetic material into cells, but there is a theoretical risk that these viruses could activate cancer genes. The FDA has suggested lifelong monitoring for patients receiving CAR-T cell therapies and stated that the benefits of these treatments still outweigh the potential risks. Pharmaceutical companies such as Novartis, Bristol Myers Squibb, and Johnson & Johnson, which manufacture approved CAR-T cell therapies, have not found evidence linking their products to secondary malignancies. However, experts acknowledge the need for more information and caution that the occurrence of such cases is likely to be rare.
- A Cutting-Edge Cancer Treatment May Cause Cancer. The FDA Is Investigating WIRED
- Promising CART T cancer therapy invented at Penn linked to rare, secondary cancers, FDA says 6abc Philadelphia
- FDA investigating risk of secondary cancers after CAR-T therapy to treat cancer CNN
- CAR-T, Lifesaving Cancer Treatment, May Sometimes Cause Cancer, FDA Says The New York Times
- As FDA probes CAR-T safety, expert meeting for Bristol's Abecma could serve as key guidepost FiercePharma
Reading Insights
0
1
2 min
vs 3 min read
80%
597 → 122 words
Want the full story? Read the original article
Read on WIRED