"FDA Approves New COVID Prophylactic for Immunocompromised Individuals"
TL;DR Summary
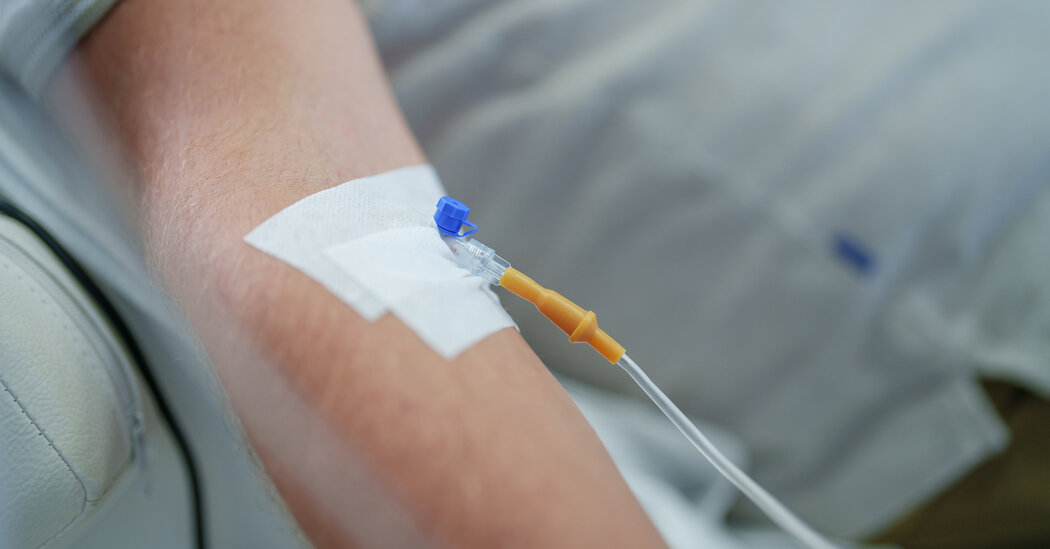
The FDA has authorized Pemgarda, a new monoclonal antibody infusion, for emergency use in immunocompromised individuals aged 12 and older who are unlikely to mount an adequate immune response after vaccination. This includes those who have received organ transplants or are undergoing cancer treatment. Pemgarda will be available in the next week or two and is administered as an infusion in healthcare settings. The drug aims to protect a small but vital group of individuals who are most at risk from Covid-19.
- F.D.A. Authorizes New Drug to Protect High-Risk Patients From Covid The New York Times
- FDA Authorizes Pemgarda for Pre-Exposure Prevention of COVID-19 in Certain Immunocompromised Individuals Infectious Disease Special Edition
- Invivyd's COVID prophylactic scores FDA emergency nod for people with weakened immune systems FiercePharma
- New Monoclonal Authorized to Prevent COVID in Immunocompromised People Medpage Today
- FDA authorizes new drug to protect immune compromised from Covid-19 STAT
Reading Insights
Total Reads
0
Unique Readers
7
Time Saved
1 min
vs 2 min read
Condensed
78%
371 → 82 words
Want the full story? Read the original article
Read on The New York Times