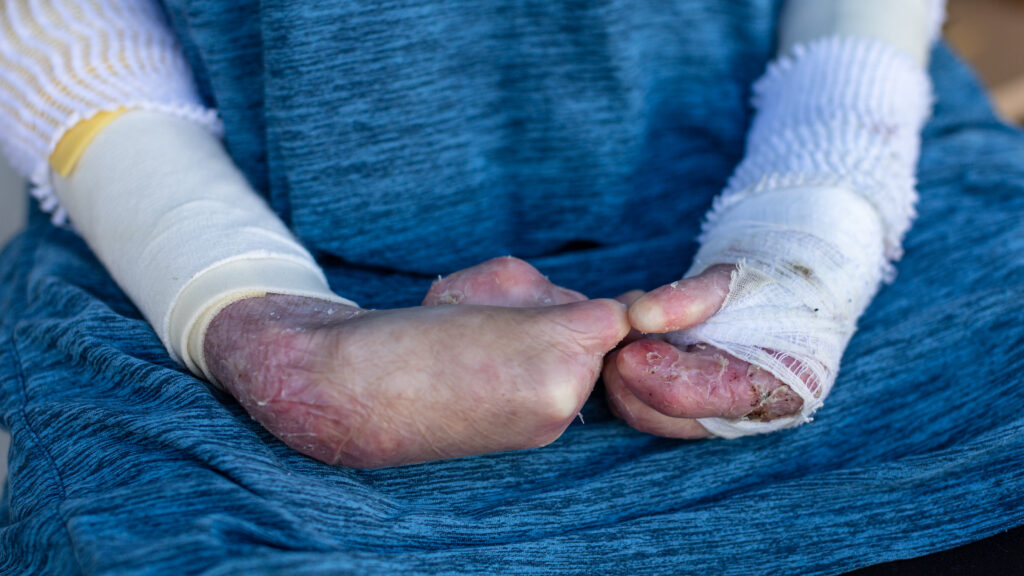
FDA approves groundbreaking gene therapy for rare skin condition.
The FDA has approved Vyjuvek, a redosable gene therapy made by Krystal Biotech, for patients with dystrophic epidermolysis bullosa, a genetic disease that causes painful blisters and persistent wounds. The topical treatment delivers a healthy copy of the gene that encodes the protein type VII collagen to the targeted skin cells, enabling healing to occur. Patients with the condition live covered in bandages to try to stop new blisters from forming and to dress their wounds.


