FDA approves groundbreaking gene therapy for rare skin condition.
TL;DR Summary
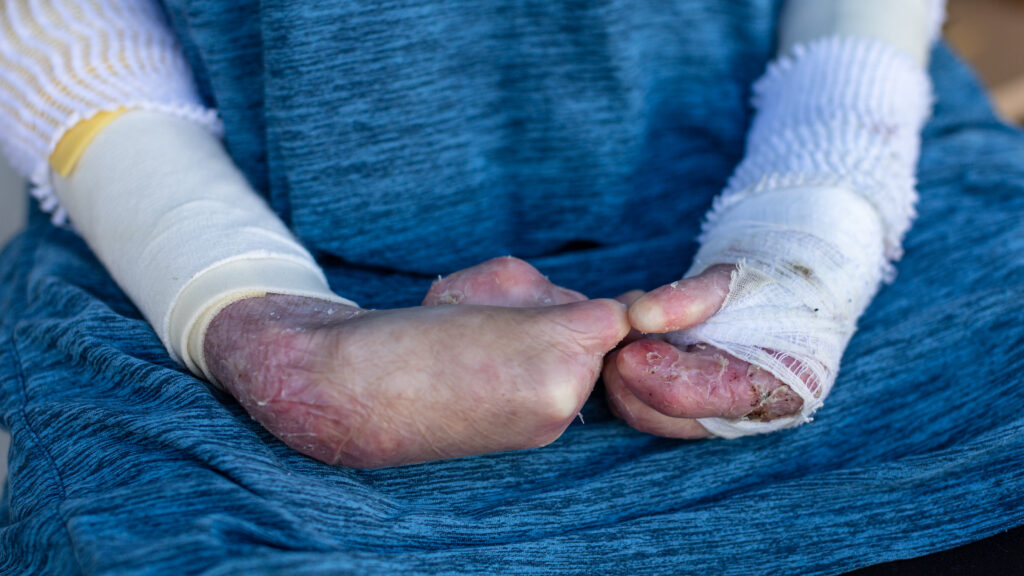
The FDA has approved Vyjuvek, a redosable gene therapy made by Krystal Biotech, for patients with dystrophic epidermolysis bullosa, a genetic disease that causes painful blisters and persistent wounds. The topical treatment delivers a healthy copy of the gene that encodes the protein type VII collagen to the targeted skin cells, enabling healing to occur. Patients with the condition live covered in bandages to try to stop new blisters from forming and to dress their wounds.
- FDA approves first treatment for skin condition that causes persistent wounds, a redosable gene therapy STAT
- Krystal's Vyjuvek becomes first topical gene therapy with FDA nod to treat rare skin disease FiercePharma
- Rare Skin Blistering Condition Gets First Drug Approved Medpage Today
- Landmark victory for individuals living with DEB and their families, the FDA announced their approval of Krystal Biotech's VYJUVEK™ for the treatment of Dystrophic Epidermolysis Bullosa (DEB). Business Wire
- FDA Approves First-Ever Topical Gene Therapy Medscape
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
1 min
vs 2 min read
Condensed
66%
221 → 76 words
Want the full story? Read the original article
Read on STAT