CDC Advises Pfizer Maternal RSV Vaccine for Infant Protection Amid Tripledemic Threat
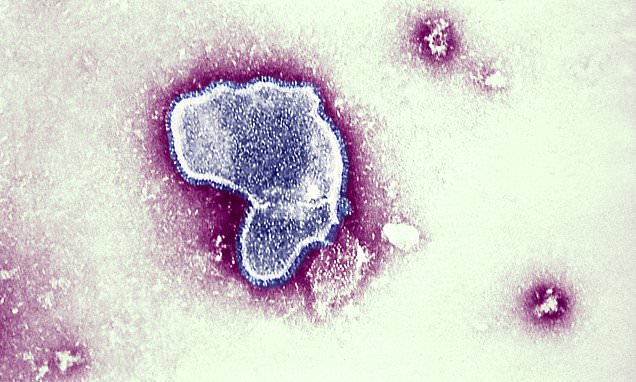
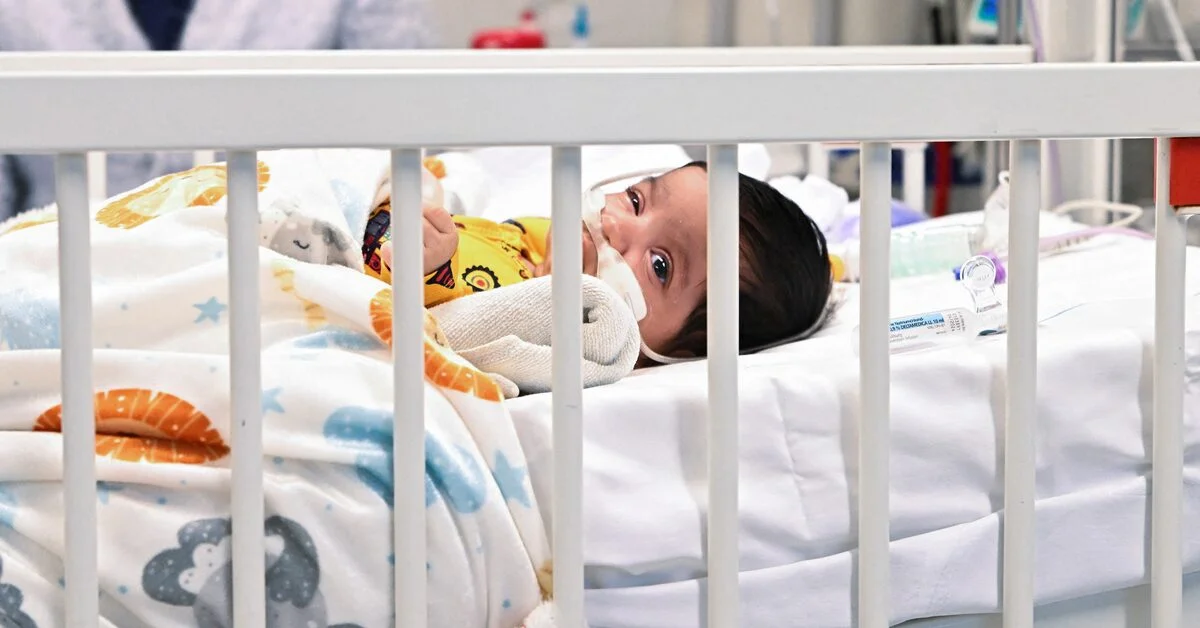
The Centers for Disease Control and Prevention's advisory panel has recommended Pfizer's maternal vaccine, Abrysvo, to protect infants from respiratory syncytial virus (RSV), the leading cause of hospitalization among babies in the U.S. The vaccine is to be administered to expectant mothers between 32 to 36 weeks into their pregnancy during September through January. The recommendation is pending approval from CDC Director Mandy Cohen. Pfizer's vaccine is the first RSV treatment to use maternal immunization, providing infants with protection against the virus from birth through the first six months of life. The panel's recommendation comes as RSV begins to spread at higher levels, and public health officials hope the vaccine will help combat the virus this fall and winter.