"FDA Advisers Tackle Ethical and Logistical Challenges of Artificial Womb Technology"
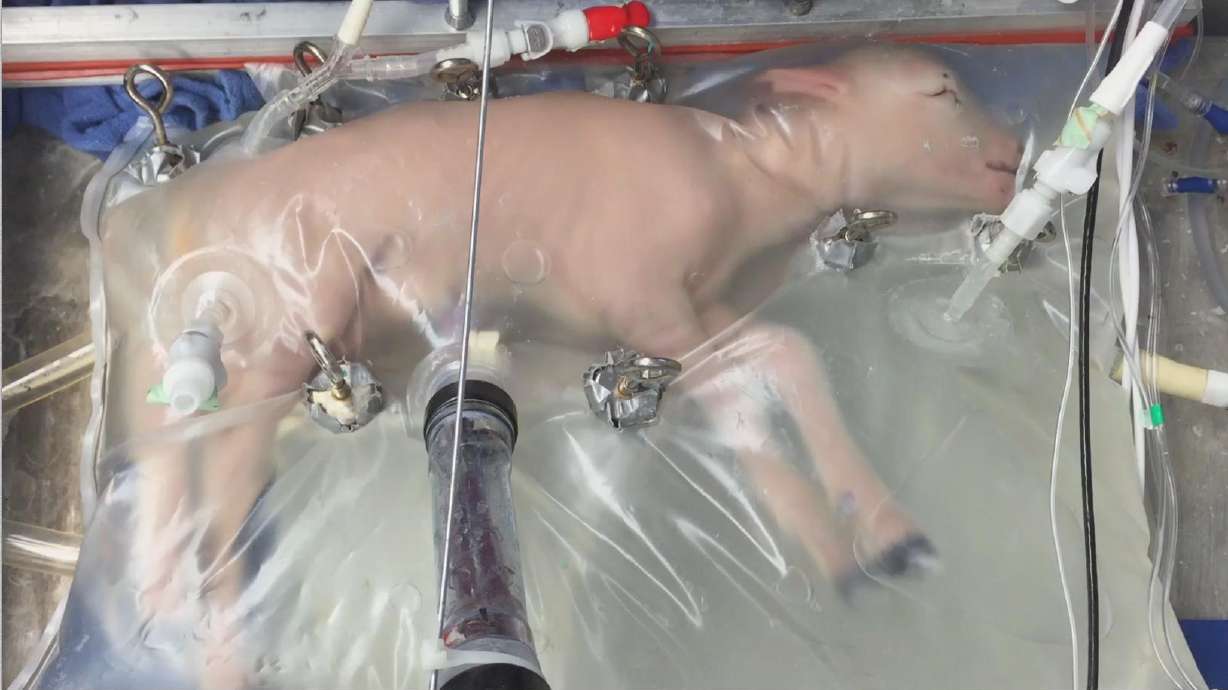
Independent advisers to the U.S. Food and Drug Administration (FDA) are discussing the regulations, ethics, and possibilities of creating an artificial womb to increase the survival chances of extremely premature babies without long-term health problems. While no such device has been tested in humans, similar ones have been used successfully in animals. Prematurity is the leading cause of death in children under 5, and an artificial womb could help address this issue. The FDA committee is considering the data and regulations required for human trials, as well as ethical considerations and long-term effects. The FDA makes its own decisions and is not bound by the advisers' recommendations.
- FDA advisers discuss future of 'artificial womb' for human infants KSL.com
- Human trials of artificial wombs could start soon. Here's what you need to know Nature.com
- FDA panel to weigh safety of artificial wombs for preterm births STAT
- ethical challenges of an artificial womb The Boston Globe
- FDA Advisors Grapple With Logistical, Ethical Issues of Artificial Womb Technology Medpage Today
Reading Insights
0
9
4 min
vs 5 min read
88%
870 → 107 words
Want the full story? Read the original article
Read on KSL.com