New ALS drug breakthrough may revolutionize brain treatments.
TL;DR Summary
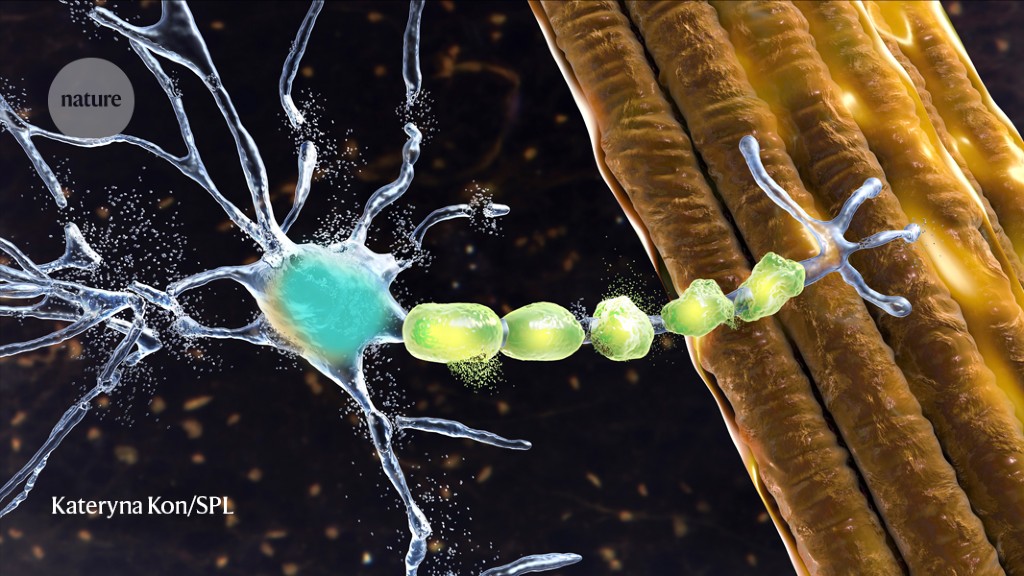
The US FDA is expected to rule soon on the approval of a new drug for a rare form of ALS, which could pave the way for more flexible regulation of neurological drugs. Tofersen, developed by Biogen and Ionis Pharmaceuticals, did not slow patients' decline in a phase III trial, but some say the trial was too short. Biogen has asked the FDA to approve the drug on an 'accelerated' basis, with future trial data to determine whether it works. The FDA's willingness to review and discuss the tofersen data highlights its newfound flexibility in neurological drug development.
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
6 min
vs 7 min read
Condensed
92%
1,276 → 98 words
Want the full story? Read the original article
Read on Nature.com