Affordability concerns arise over FDA approval of over-the-counter Narcan sales.
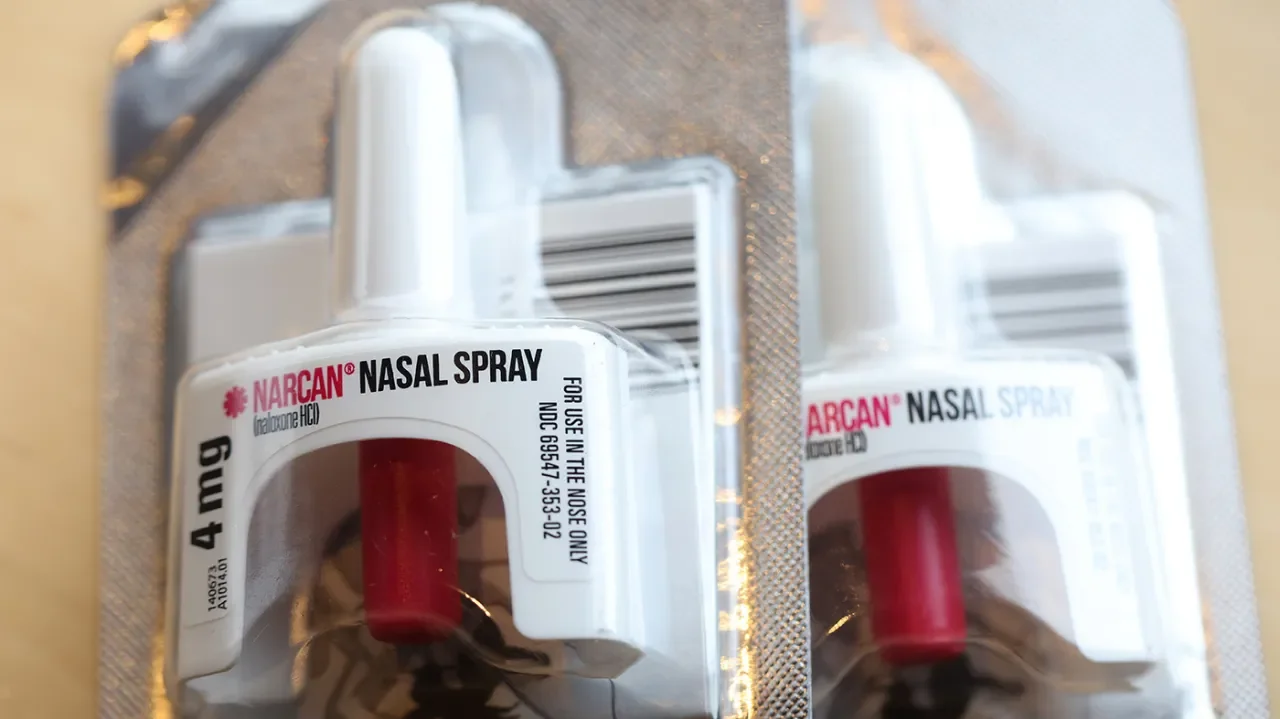
The FDA has approved Narcan, a brand-name nasal spray manufactured by Emergent BioSolutions, to be sold over-the-counter without a prescription. However, there is concern that the drug may not be affordable for those who need it most. The cost of the medication, requirements to show ID, and the overall stigma of asking for naloxone are all barriers. Health insurance plans don’t typically cover over-the-counter drugs, so there’s a chance FDA’s move could make Narcan more expensive than it already is. The hope is that making Narcan available over-the-counter will eliminate some of the “bureaucratic gatekeeping” that prevents harm reduction groups from purchasing and distributing Narcan.
- Narcan approval could be a game changer — if people can afford it The Hill
- How FDA approval of over-the-counter Narcan sales affects the opioid crisis PBS NewsHour
- CUPHD, Paxton pharmacist respond to Narcan over-the-counter FDA approval wcia.com
- St. Joseph Co. Dept. of Health reacts to FDA decision to approve over-the-counter Narcan WNDU
- Nebraska pharmacists and addiction specialists question future availability of over-the-counter Narcan WOWT
Reading Insights
0
1
4 min
vs 5 min read
89%
980 → 105 words
Want the full story? Read the original article
Read on The Hill