FDA recalls Roche and SD Biosensor COVID-19 at-home tests over bacterial contamination risk.
TL;DR Summary
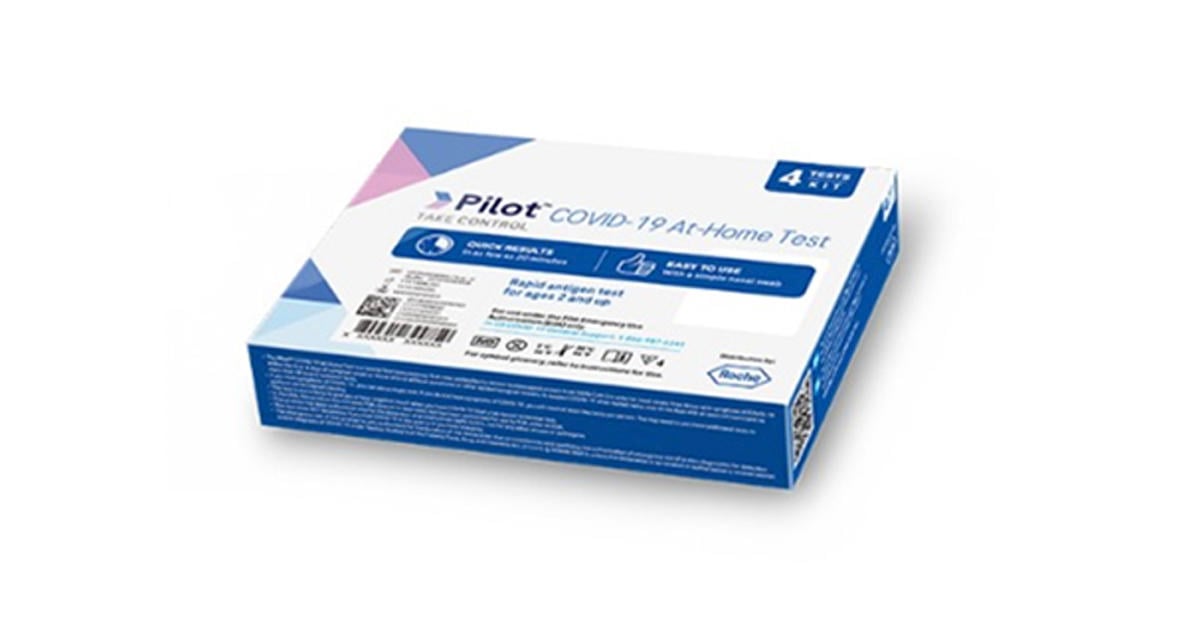
The FDA has issued a recall for over 500,000 "Pilot COVID-19 At-Home Tests" from Roche and SD Biosensor due to concerns over bacterial contamination in the liquid buffer solution. The recalled tests were distributed to CVS and Amazon, but not through the federal government's testing programs. The bacteria found in the tests can lead to potentially dangerous infections, especially in people with compromised immune systems. So far, no injuries or deaths have been reported, but people who have already used the tests may have received false positive or false negative results. Roche and SD Biosensor are cooperating with the FDA in their investigation.
- COVID test recall: FDA warns of bacterial contamination in some Pilot COVID-19 At-Home Tests from Roche and SD Biosensor CBS News
- FDA warns that half a million COVID tests from Roche and SD Biosensor should be thrown out CBS Miami
- Certain SD Biosensor Pilot COVID-19 at-home tests recalled due to potential contamination News 12 Bronx
- FDA recalls some at-home COVID-19 tests over possible bacteria risk WBTV
- Some Pilot COVID-19 At Home Tests recalled by FDA WTVR CBS 6
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
2 min
vs 3 min read
Condensed
75%
404 → 103 words
Want the full story? Read the original article
Read on CBS News