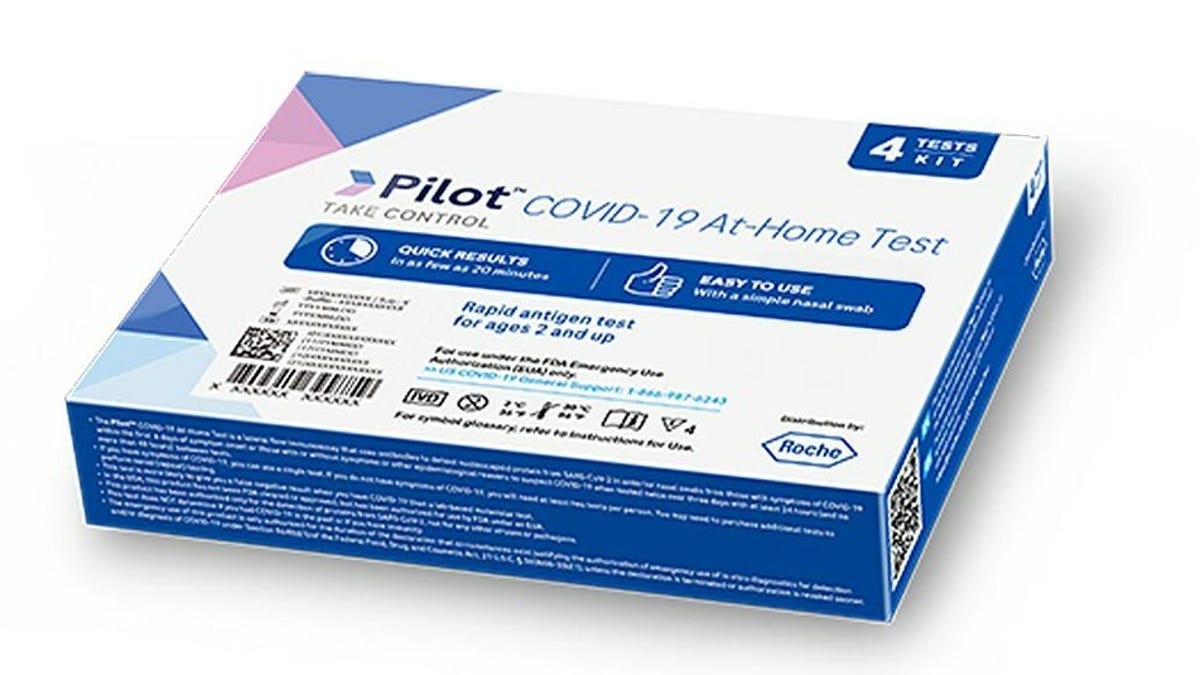
FDA recalls certain COVID-19 at-home tests over contamination concerns.
TL;DR Summary
The FDA has issued a recall for certain lots of SD Biosensor, Inc. Pilot COVID-19 At-Home Tests distributed by Roche Diagnostics due to bacterial contamination that may lead to infection. The recall affects 44 lot numbers and consumers who have the affected kits should throw them away entirely. The liquid solution should not be poured down the drain. The FDA is working with SD Biosensor to resolve the situation and assess corrective actions.
- Food and Drug Administration recalls some Pilot COVID-19 at-home tests USA TODAY
- Certain SD Biosensor Pilot COVID-19 at-home tests recalled due to potential contamination News 12 Bronx
- Some Pilot COVID-19 At Home Tests recalled by FDA WTVR CBS 6
- Some COVID-19 At Home Tests recalled by FDA over bacteria risk CBS Miami
- COVID-19: Do Not Use These At-Home Tests Due To Bacteria Concerns, FDA Says Daily Voice
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
1 min
vs 2 min read
Condensed
77%
317 → 73 words
Want the full story? Read the original article
Read on USA TODAY