FDA Panel Recommends Approval of RSV Preventive Therapy for Infants
TL;DR Summary
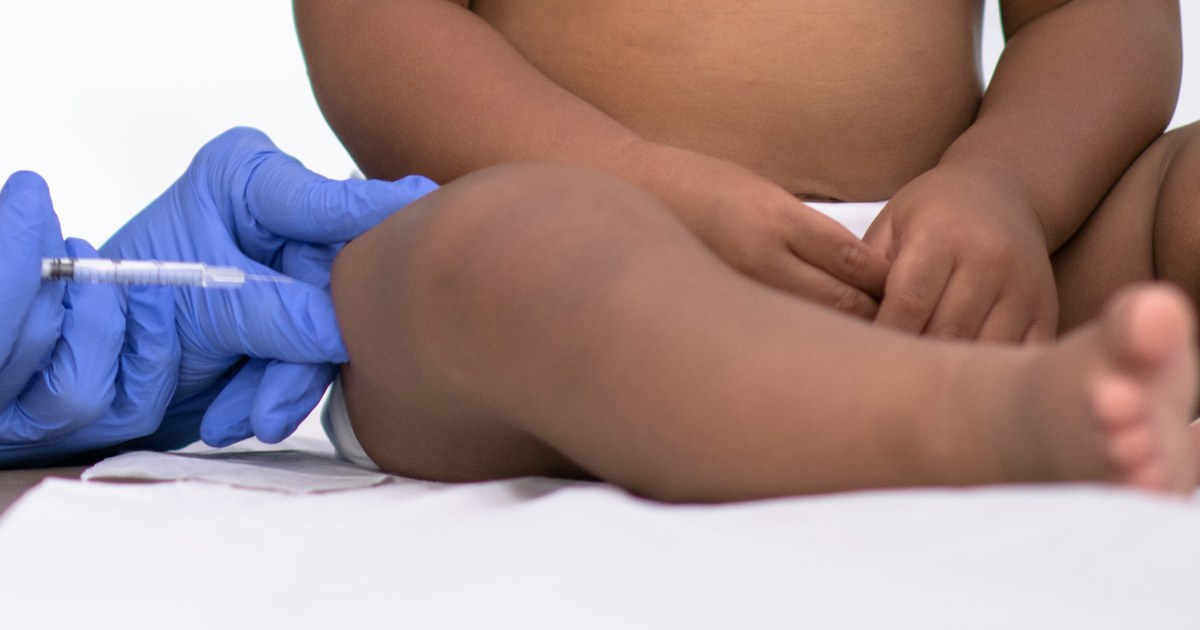
An FDA advisory committee has recommended an injectable monoclonal antibody drug called nirsevimab, which functions similarly to a vaccine, to protect infants up to 2 years old from respiratory syncytial virus (RSV). The drug, sold under the name Beyfortus, was found to lower the risk of developing respiratory disease from RSV that required a doctor's visit by nearly 75% for at least five months. The FDA must now decide whether to approve the injection, but is likely to follow the committee’s recommendation. No RSV vaccine for infants has been approved yet.
- FDA panel recommends drug to prevent RSV in infants NBC News
- F.D.A. Panel Recommends R.S.V. Shot to Protect Infants The New York Times
- Sanofi, AstraZeneca's RSV antibody for infants easily clears FDA adcomm, likely setting up approval FiercePharma
- US FDA panel backs Sanofi-AstraZeneca's preventive RSV therapy Reuters
- RSV antibody drug gets backing from FDA advisors, paving the way for approval : Shots - Health News NPR
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
4 min
vs 5 min read
Condensed
90%
897 → 91 words
Want the full story? Read the original article
Read on NBC News