FDA Issues Warning After Probiotic-Related Infant Fatality
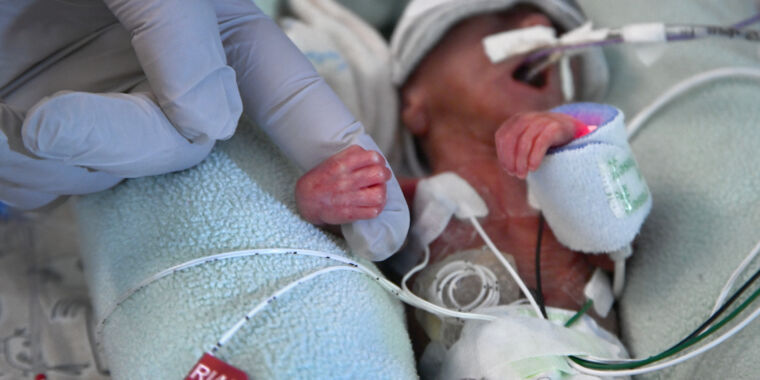
The FDA has issued a warning to healthcare providers about the use of probiotics containing live bacteria or yeast in preterm infants after a preterm, low-weight infant died from sepsis caused by a probiotic product. The product, Evivo with MCT Oil made by Infinant Health, was found to contain a genetic match to the bacterium isolated from the infant's blood. The FDA emphasized that probiotics are not approved for use as drugs or biological products in infants and highlighted the potential risks associated with their use in vulnerable populations. The agency also criticized the manufacturer for marketing the unapproved product for use in preventing serious diseases in preterm infants.
- Probiotic bacterium kills preterm infant; FDA blasts supplement maker Ars Technica
- After death of infant, FDA warns hospitals about probiotics for premature babies CNN
- FDA Investigates Probiotics in Preterm Infants After Fatal Incident PEOPLE
- Following One Death, FDA Warns Hospitals About Giving Probiotics to Preemies U.S. News & World Report
- View Full Coverage on Google News
Reading Insights
0
0
2 min
vs 3 min read
80%
539 → 109 words
Want the full story? Read the original article
Read on Ars Technica