FDA approves groundbreaking fecal transplant pill for gut health.
TL;DR Summary
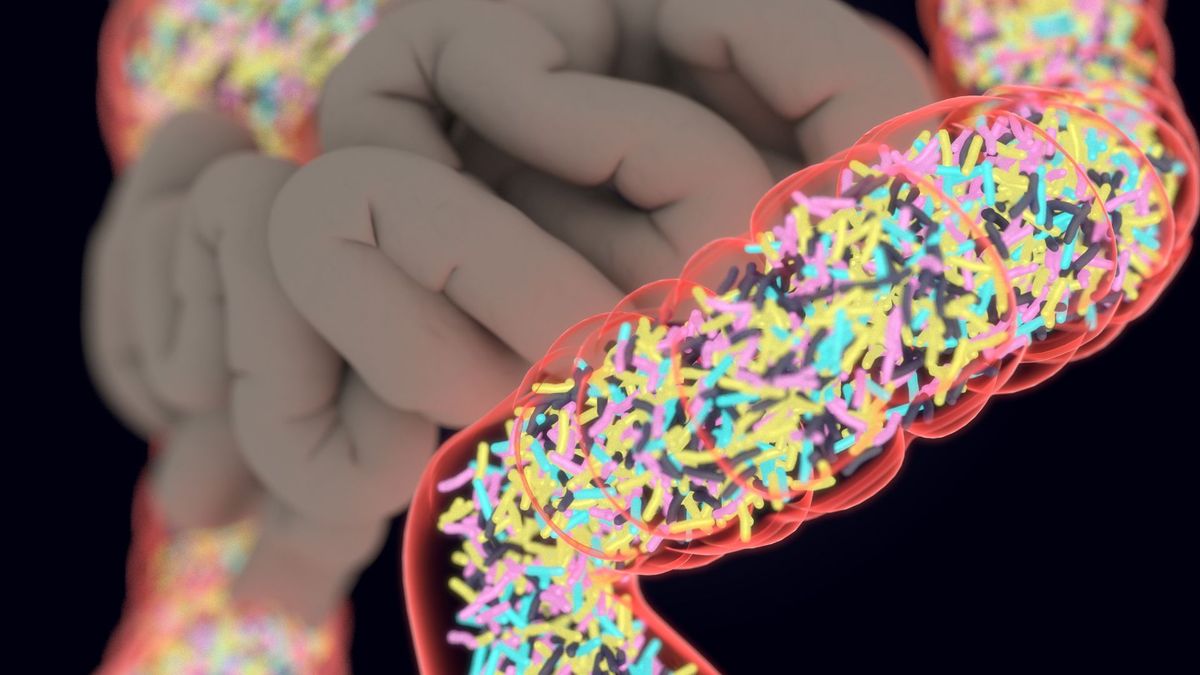
The FDA has approved the first pill made from donated human feces, called Vowst, as a preventive treatment for recurrent infections with the bacterium Clostridioides difficile. The pill contains live bacteria and is taken orally, unlike the previously approved enema-based treatment. Antibiotics can disrupt the balance of bacteria in the gut, leading to C. diff infections. Fecal microbiota products offer a new way to prevent recurrent C. diff by replenishing the gut microbiome. The Vowst treatment regimen involves taking four capsules once a day for three days in a row, starting two to four days after finishing a course of antibiotics for C. diff.
- FDA approves 1st pill made from human poop Livescience.com
- First pill for fecal transplants wins FDA approval NBC News
- FDA approves first-ever fecal transplant pill to restore gut bacteria Interesting Engineering
- Seres to start selling 'poop pill' in June at $17,500 per course - Boston Business Journal The Business Journals
- Seres's Bacteria in a Pill Becomes First FDA-Approved Oral Microbiome Therapy MedCity News
Reading Insights
Total Reads
0
Unique Readers
9
Time Saved
3 min
vs 4 min read
Condensed
84%
664 → 104 words
Want the full story? Read the original article
Read on Livescience.com