FDA approves drug for rare form of ALS.
TL;DR Summary
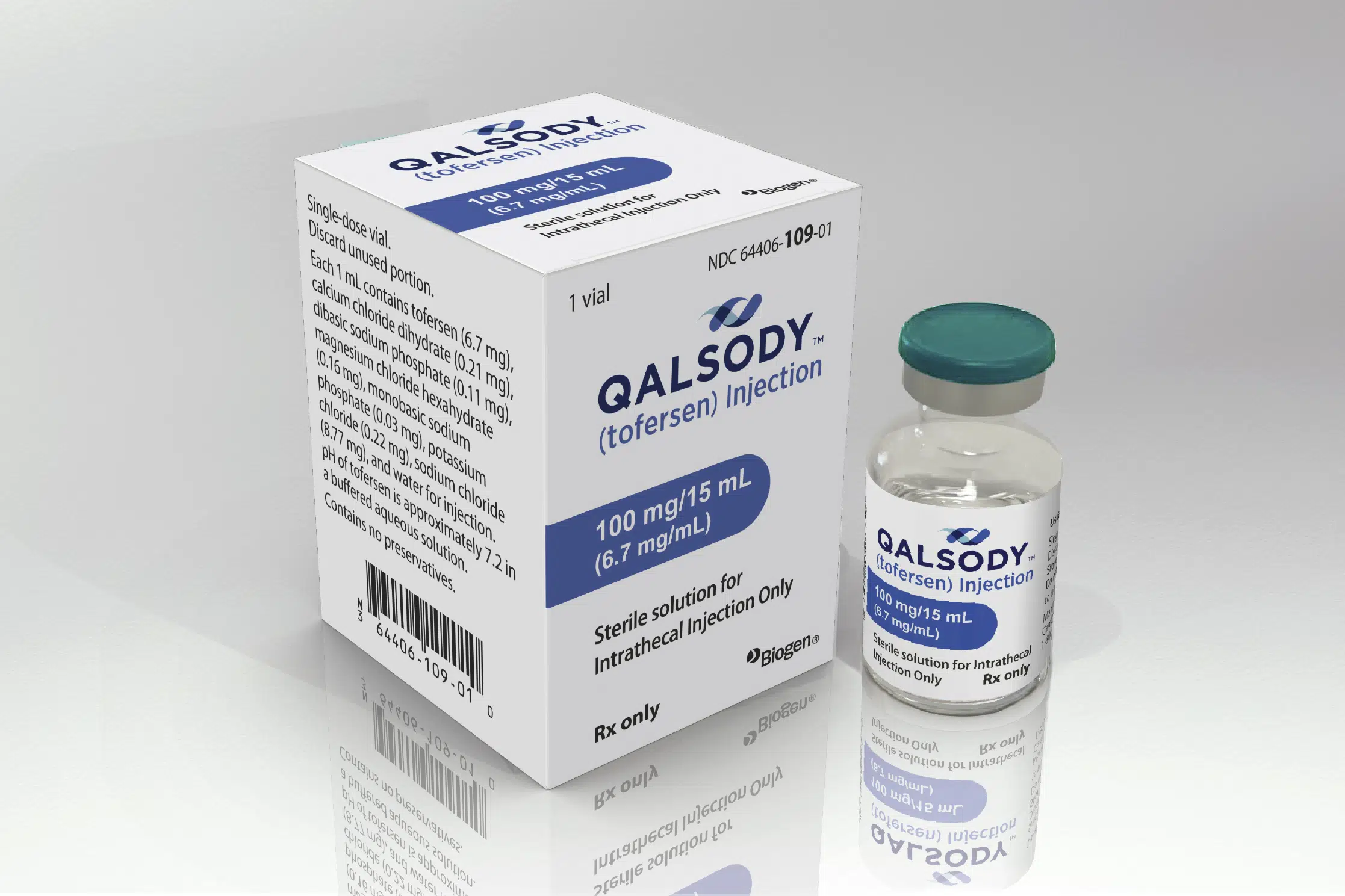
The FDA has approved Biogen's injectable drug, tofersen, for a rare genetic mutation that causes ALS, or Lou Gehrig's disease, which affects less than 500 people in the US. The drug is the first for an inherited form of ALS and was approved via the FDA's accelerated pathway, which allows drugs to launch based on promising early results. However, the FDA is requiring Biogen to continue studying the drug in a trial of people who carry the genetic mutation but do not yet have ALS symptoms.
- Drug for rare form of ALS approved by FDA The Associated Press
- FDA grants accelerated approval for Biogen ALS drug that treats rare form of the disease CNBC
- F.D.A. Approves Drug for Rare Form of A.L.S. The New York Times
- Biogen Stock Dives As ALS Win Does Little To Outweigh 'Low-Quality' Beat Investor's Business Daily
- Drug for rare form of ALS, based in part on WashU research, approved by FDA – Washington University School of Medicine in St. Louis Washington University School of Medicine in St. Louis
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
3 min
vs 4 min read
Condensed
89%
757 → 86 words
Want the full story? Read the original article
Read on The Associated Press