FDA approves Cidara's Rezzyao for candidiasis treatment despite company's Q4 earnings disappointment.
TL;DR Summary
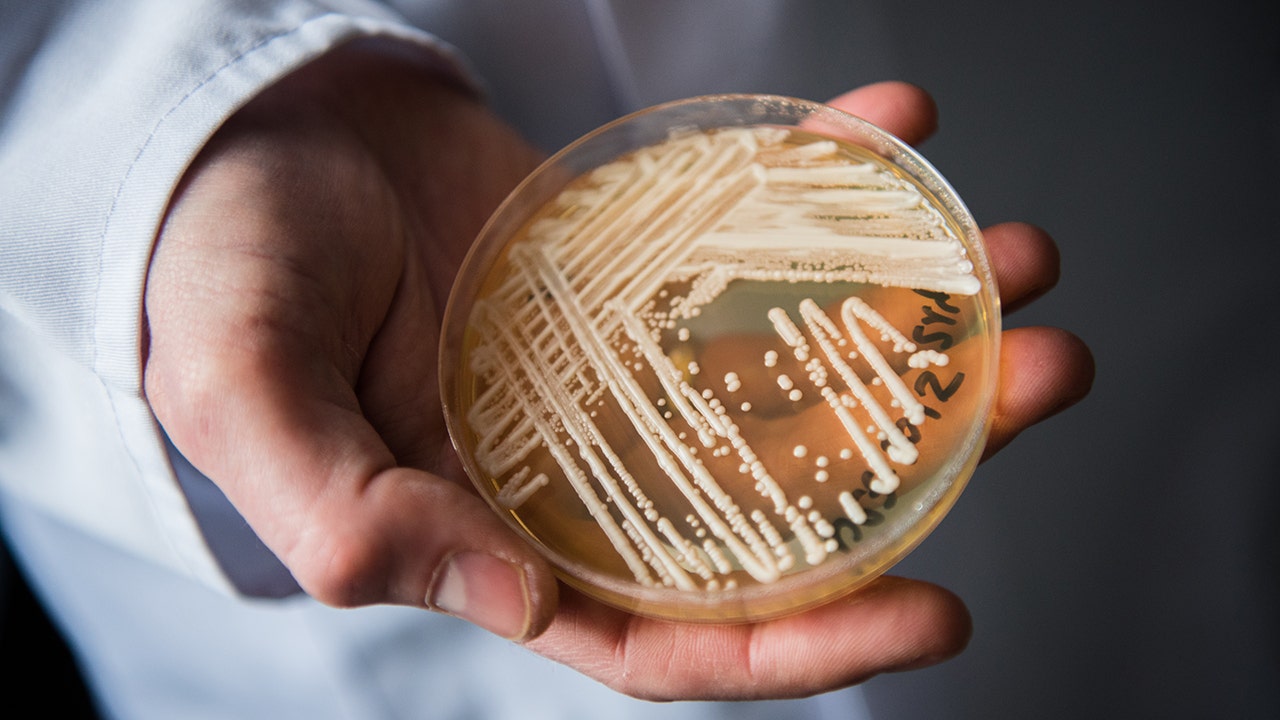
The FDA has approved an antifungal drug called Rezzayo, developed by Cidara Therapeutics and Melinta Therapeutics, for the treatment of candidemia and invasive candidiasis caused by Candida species, including the drug-resistant Candida auris. Rezzayo is the first new treatment option approved for these deadly fungal infections in over a decade. Candida auris is an antimicrobial resistance threat that has spread rapidly throughout the US in 2020 and 2021, and is often resistant to multiple antifungal drugs, making it a superbug.
- Deadly fungus and companies with potential treatment Fox Business
- FDA signs off on Cidara's antifungal Rezzayo for candidiasis FiercePharma
- Cidara's Lower Than Expected Q4 Earnings Overshadows FDA Nod For Antifungal Treatment Yahoo Finance
- FDA Approves Long-Acting Antifungal for Treating Candida Medpage Today
- Why Is Cidara Therapeutics (CDTX) Stock Down 12% Today? InvestorPlace
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
3 min
vs 4 min read
Condensed
87%
629 → 80 words
Want the full story? Read the original article
Read on Fox Business