FDA approves Cidara's Rezzyao for candidiasis treatment despite company's Q4 earnings disappointment.
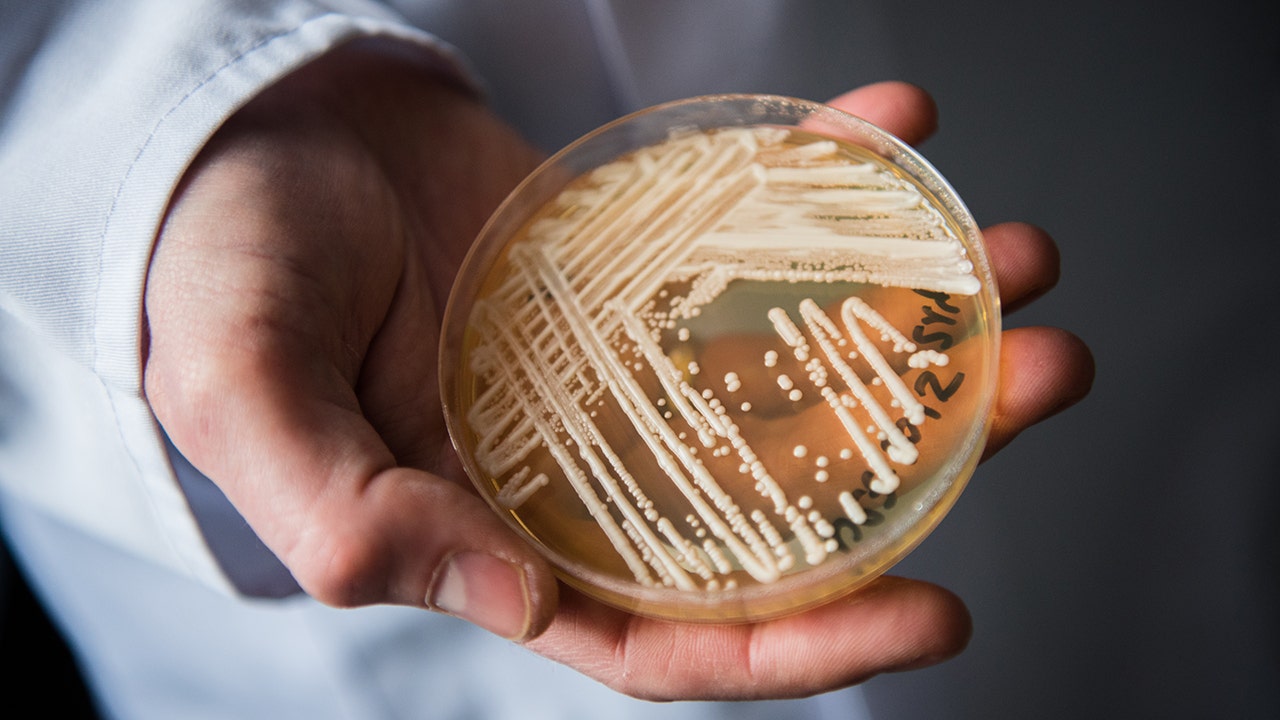
The FDA has approved an antifungal drug called Rezzayo, developed by Cidara Therapeutics and Melinta Therapeutics, for the treatment of candidemia and invasive candidiasis caused by Candida species, including the drug-resistant Candida auris. Rezzayo is the first new treatment option approved for these deadly fungal infections in over a decade. Candida auris is an antimicrobial resistance threat that has spread rapidly throughout the US in 2020 and 2021, and is often resistant to multiple antifungal drugs, making it a superbug.
