FDA-approved breast cancer drug reduces risk of recurrence by 25%.
TL;DR Summary
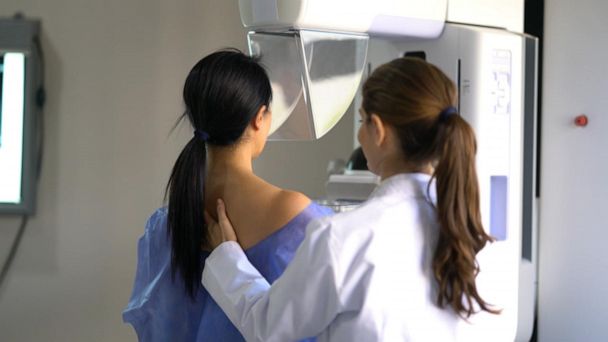
Novartis has announced that its breast cancer treatment Kisqali, already approved by the FDA for advanced stages of breast cancer, can reduce the risk of cancer coming back when added after primary treatment. The drug could be good news for women diagnosed in the earlier stages of the disease and those who are hormone-receptor positive and HER2 negative, who make up 70% of the breast cancer population. The findings were announced at the annual meeting of the American Society of Clinical Oncology, and Novartis plans to submit the data to regulatory authorities in the US and Europe before the end of the year.
- FDA-approved drug found to reduce risk of breast cancer coming back GMA
- Novartis CEO Vas Narasimhan on the latest research on a breast cancer drug CNBC Television
- Drug cuts risk of breast cancer recurrence for some early-stage patients, trial shows CNN
- Breast cancer drug cuts risk of most common form returning by 25% The Guardian
- Novartis drug cuts recurrence risk by 25% in early-stage breast cancer Reuters
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
2 min
vs 3 min read
Condensed
77%
452 → 103 words
Want the full story? Read the original article
Read on GMA