Doctors Remove Large Hairball From Girl After Years of Hair Eating
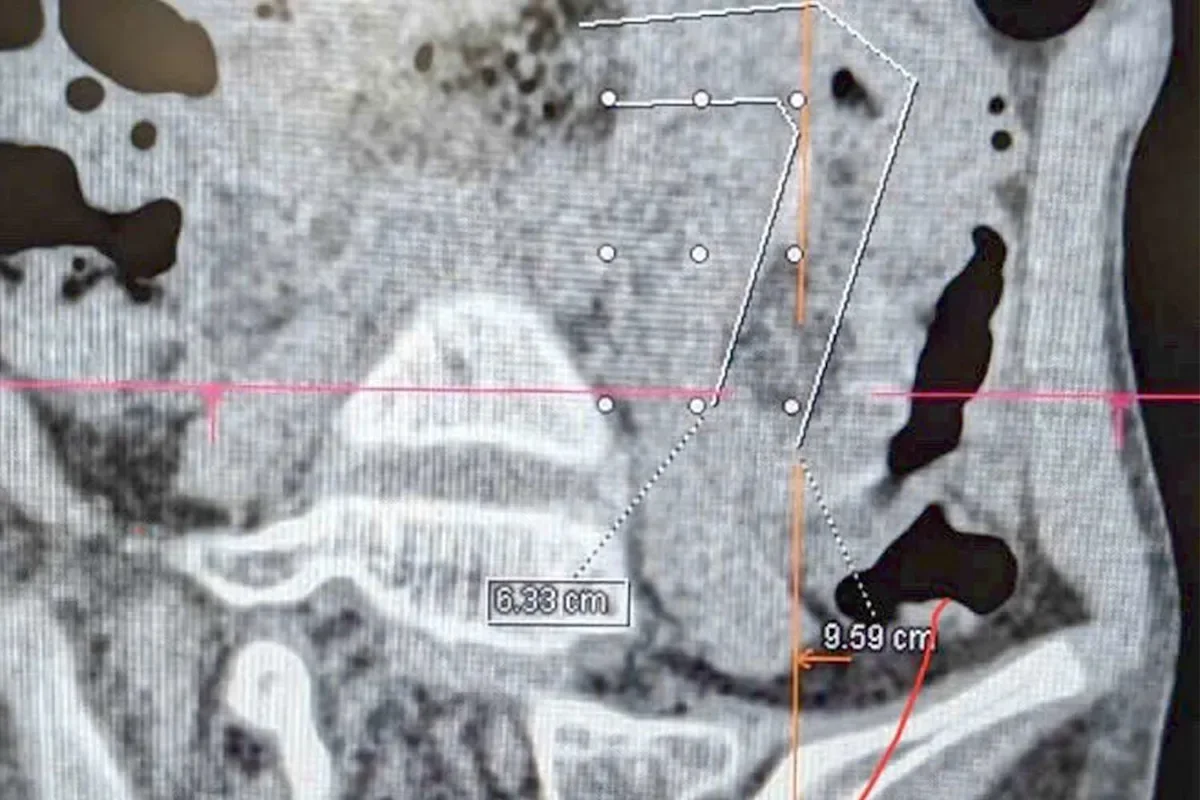
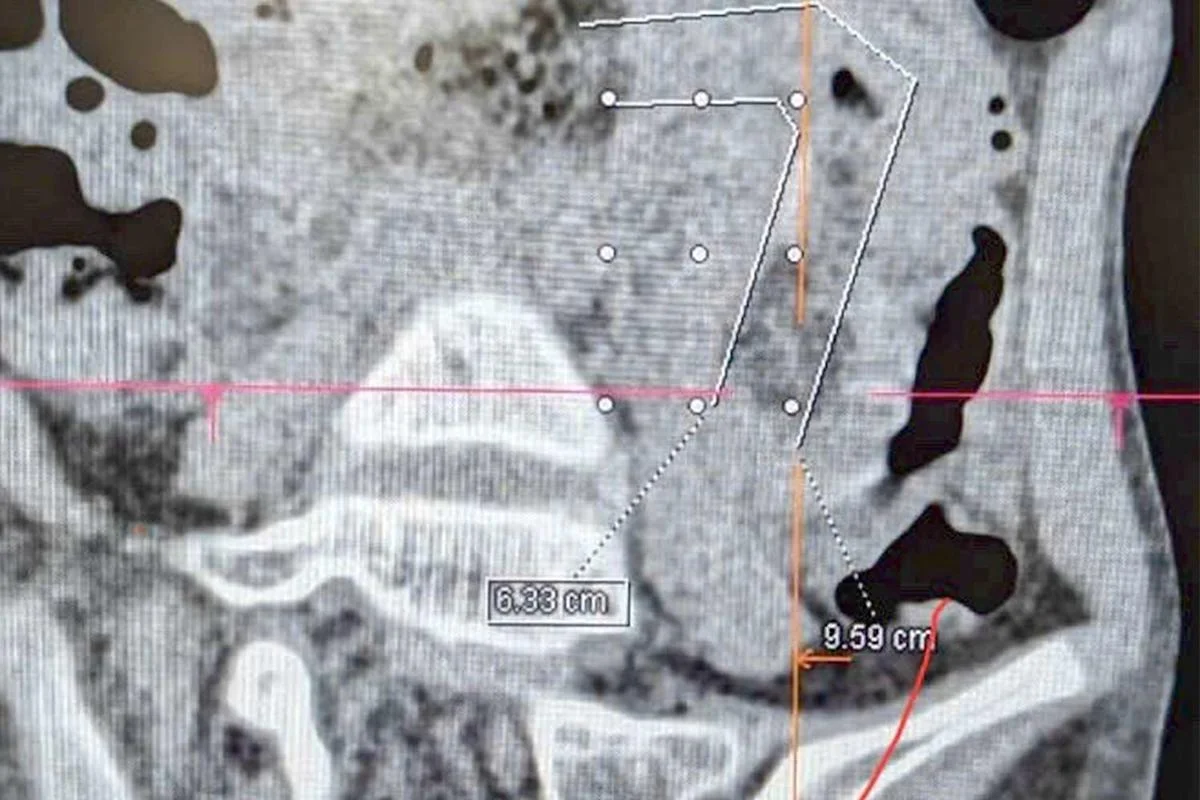
A 9-year-old girl in Vietnam was hospitalized after a massive hairball caused a complete intestinal blockage, traced back to years of undetected hair eating linked to trichophagia. She underwent minimally invasive surgery and recovered well, highlighting the importance of early psychological intervention for such behaviors.