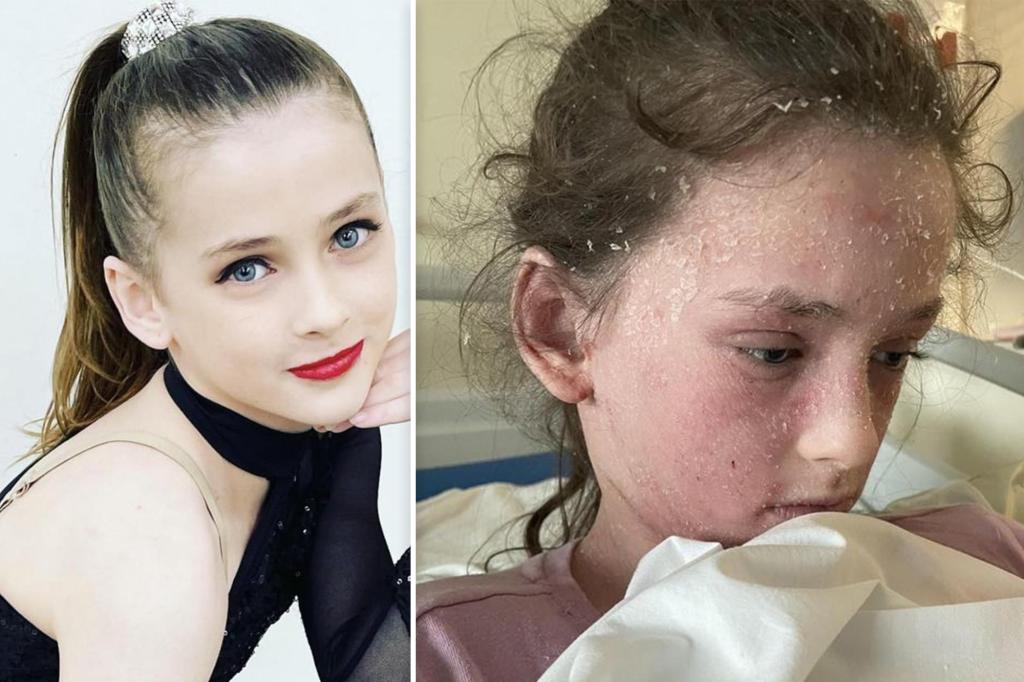
Corvus' eczema pill shows early promise with strong skin-improvement signals
Corvus Pharmaceuticals reports encouraging early-stage results for its oral eczema treatment soquelitinib: after eight weeks, 75% of treated patients reached EASI-75 and 33% achieved clear or almost clear skin versus 20% and none on placebo, suggesting potential superiority to Dupixent, though data are preliminary and from a small trial.