FDA Investigates Safety Concerns Over Dental Devices
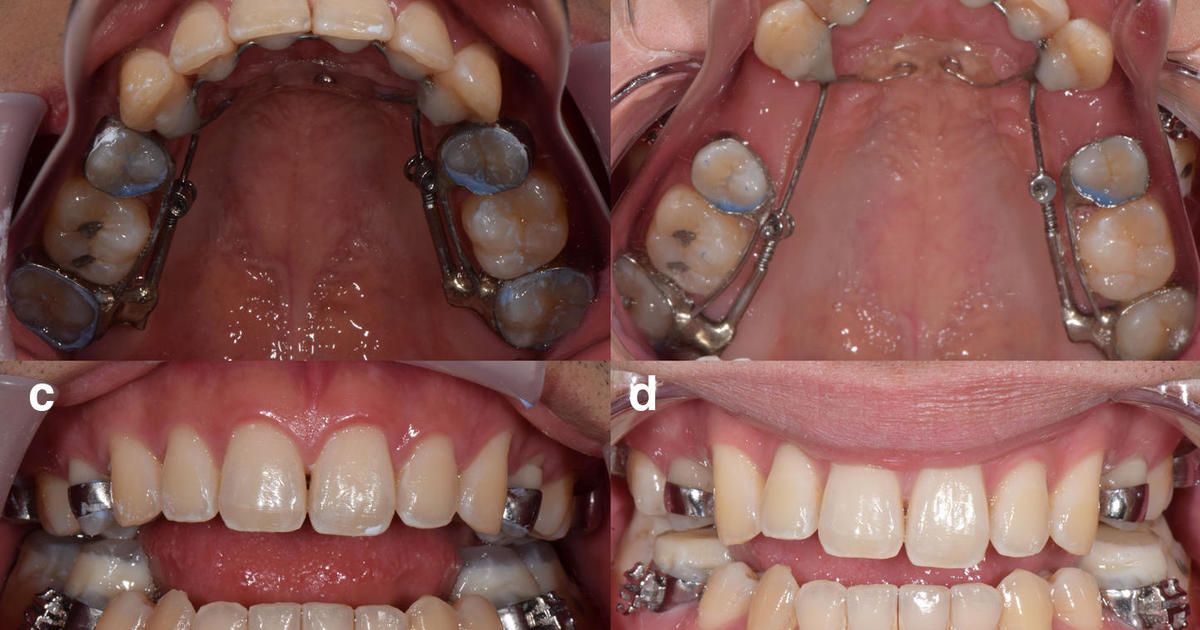
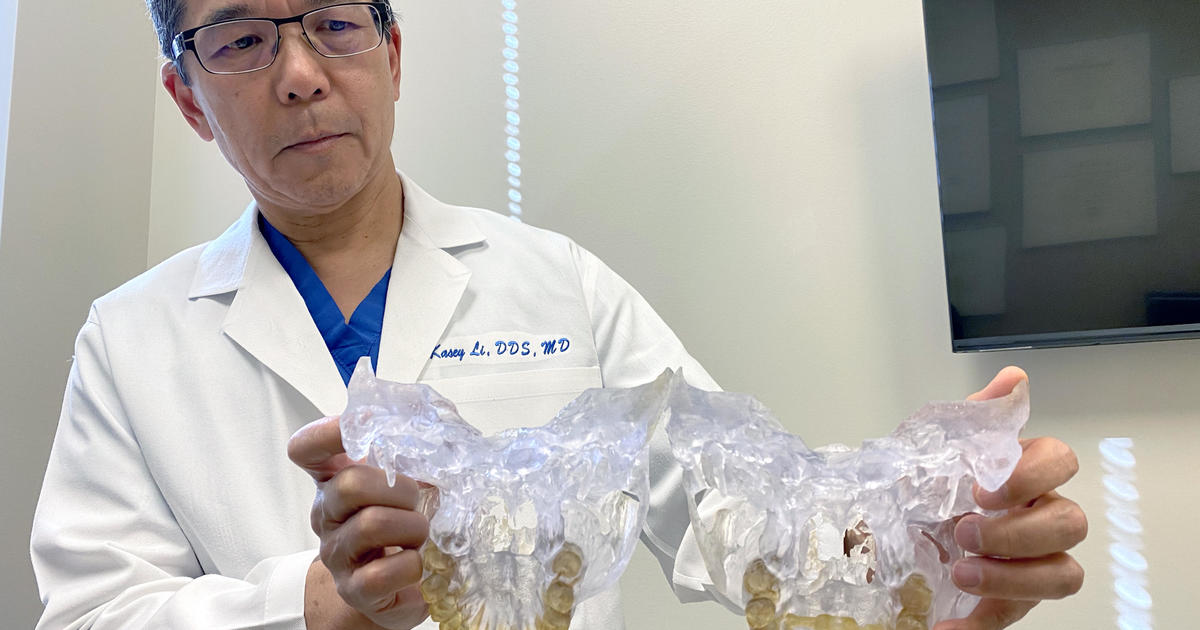
The FDA is "evaluating safety concerns" over dental devices, including the Anterior Growth Guidance Appliance (AGGA), which has been fitted on more than 10,000 dental patients and is the subject of multiple lawsuits alleging serious harm. The FDA is also looking at other similar devices, including the Anterior Remodeling Appliance (ARA). The devices have been used to treat conditions such as sleep apnea and temporomandibular joint disorder, but the safety and effectiveness of these devices have not been established. The FDA is asking patients and healthcare providers to report any complications experienced with these devices.