FDA Investigates Safety Concerns Over Dental Devices
TL;DR Summary
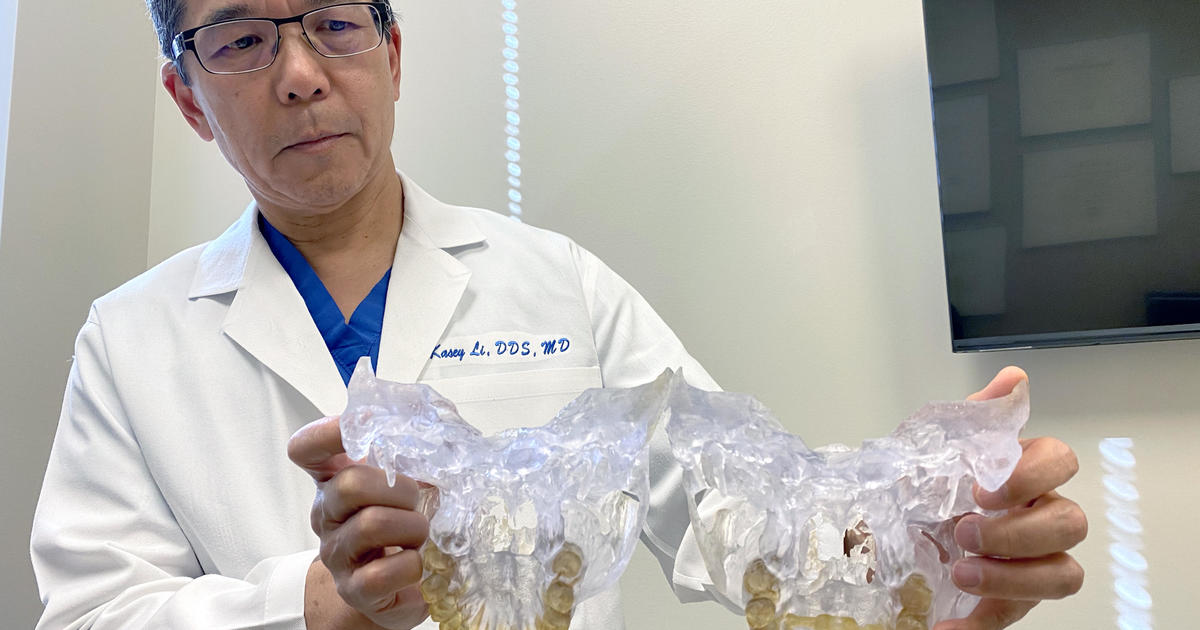
The FDA is "evaluating safety concerns" over dental devices, including the Anterior Growth Guidance Appliance (AGGA), which has been fitted on more than 10,000 dental patients and is the subject of multiple lawsuits alleging serious harm. The FDA is also looking at other similar devices, including the Anterior Remodeling Appliance (ARA). The devices have been used to treat conditions such as sleep apnea and temporomandibular joint disorder, but the safety and effectiveness of these devices have not been established. The FDA is asking patients and healthcare providers to report any complications experienced with these devices.
- FDA announces it's "evaluating safety concerns" over dental devices used by thousands CBS News
- FDA begins investigation of unauthorized dental device that sparked at least 20 lawsuits FierceBiotech
- Readers and Tweeters Are Horrified by Harm Tied to Dental Device Kaiser Health News
- FDA Evaluates 'Safety Concerns' Over Dental Devices Featured in KHN-CBS Investigation Kaiser Health News
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
5 min
vs 6 min read
Condensed
91%
1,007 → 95 words
Want the full story? Read the original article
Read on CBS News