CDC Expedites Release of Thousands of RSV Shots for Infants Amid Shortage
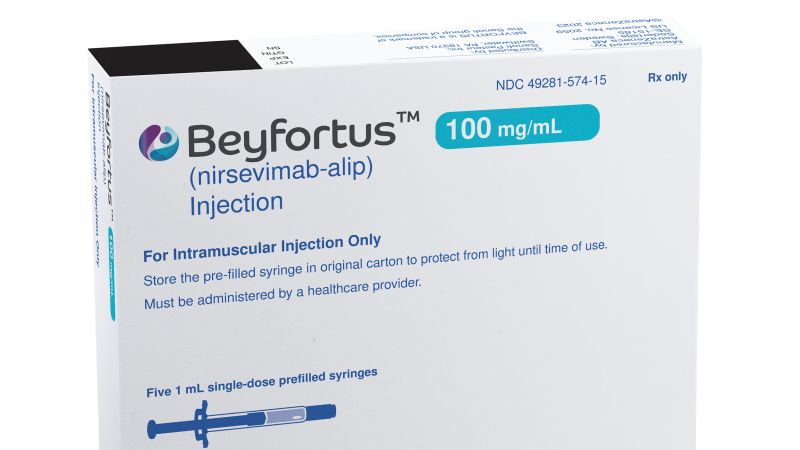
The US Centers for Disease Control and Prevention (CDC) has announced the expedited distribution of over 77,000 additional doses of nirsevimab, an RSV immunization for infants, to address the ongoing shortage. The monoclonal antibody, marketed as Beyfortus, helps protect infants from severe respiratory syncytial virus (RSV) infections, which are a leading cause of hospitalization in infants. The CDC and FDA will work closely with manufacturers to ensure the availability of more doses through the end of the year and early next year. The shortage has raised concerns among public health officials as RSV cases rise during the winter season. Challenges include limited access to the vaccine in birthing hospitals and the high cost of the immunization. Efforts are underway to address these issues and improve access in future seasons.
- Thousands more doses of RSV shot for infants expedited for release amid shortage CNN
- CDC releases 77000 more doses of RSV shot for infants The Washington Post
- RSV is straining some hospitals, and US officials are releasing more shots for newborns Fox News
- RSV: CDC expedites release of Sanofi, AstraZeneca drug amid shortage CNBC
- CDC releases more RSV vaccines as cases spike in some states KOMO News
Reading Insights
0
6
5 min
vs 6 min read
88%
1,087 → 129 words
Want the full story? Read the original article
Read on CNN