X-rays reveal the breaking of nature's strongest bond.
TL;DR Summary
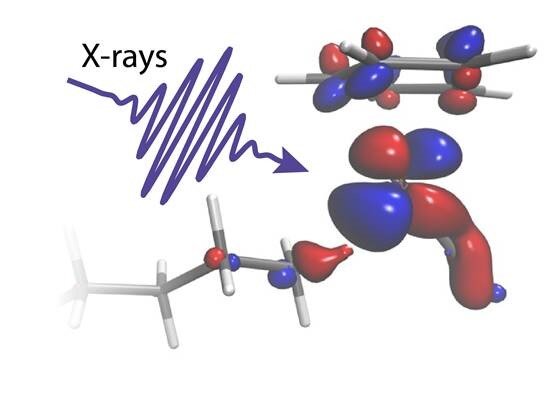
Scientists have used X-ray flashes to reveal how carbon-hydrogen bonds of alkanes break and how the catalyst works in this reaction. The research solves a 40-year-old mystery about how an activated catalyst can actually break strong C-H bonds by carefully exchanging fractions of electrons and without the need for huge temperatures or pressures. The researchers next want to learn how to direct the flow of electrons to help develop better catalysts for the chemical industry in order to make something useful out of methane and other alkanes.
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
3 min
vs 4 min read
Condensed
87%
682 → 87 words
Want the full story? Read the original article
Read on Phys.org