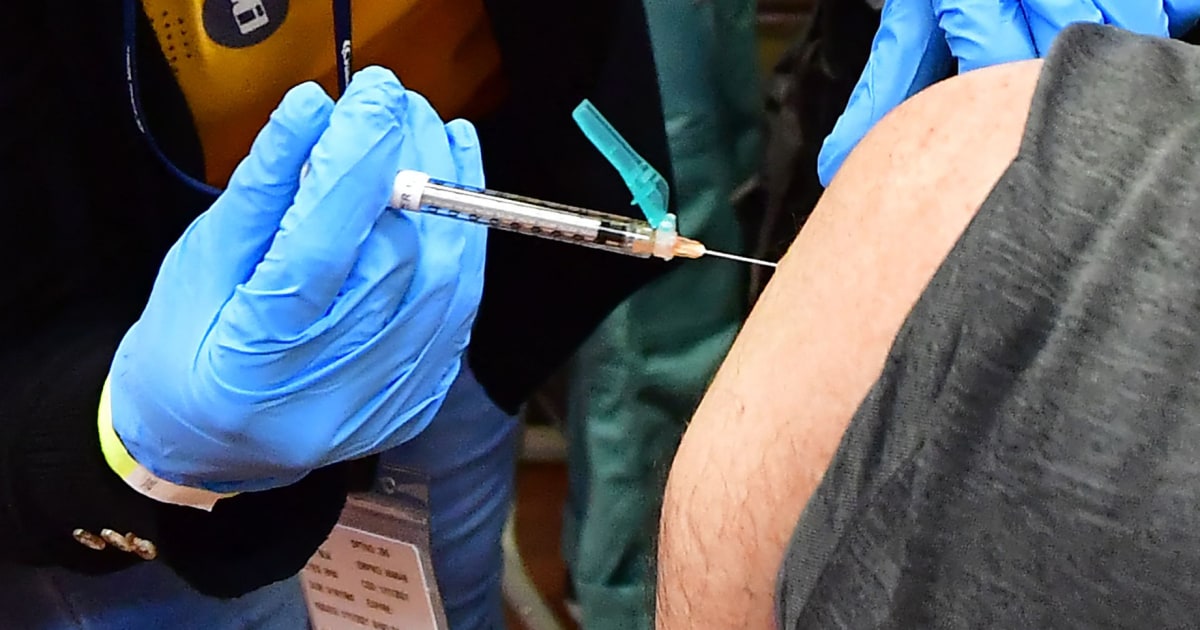
FDA approves updated Covid booster options for high-risk individuals and seniors.
TL;DR Summary
The FDA has authorized a second dose of the updated Covid booster for older adults and people with weakened immune systems. The agency also authorized using the bivalent formula in all Covid vaccines moving forward and is doing away with the multi-dose primary series for people who have not yet been vaccinated. The CDC will review the decision and if approved, immunizations could begin immediately. The booster shots were reformulated last August to target the BA.4 and BA.5 omicron subvariants, in addition to the original strain of the virus.
- FDA authorizes 2nd dose of updated Covid booster for older adults NBC News
- FDA okays second omicron booster for people at high risk from covid The Washington Post
- Single Dose of Omicron-Targeting Vaccines to Become Main Covid-19 Shot in U.S. The Wall Street Journal
- F.D.A. Authorizes Another Covid Booster Shot for People Over 65 The New York Times
- FDA: Single dose of bivalent COVID vaccine enough for most people The Hill
- View Full Coverage on Google News
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
2 min
vs 3 min read
Condensed
81%
467 → 89 words
Want the full story? Read the original article
Read on NBC News