"PANDA Trial: Neoadjuvant Atezolizumab Plus Chemotherapy for Gastric Adenocarcinoma"
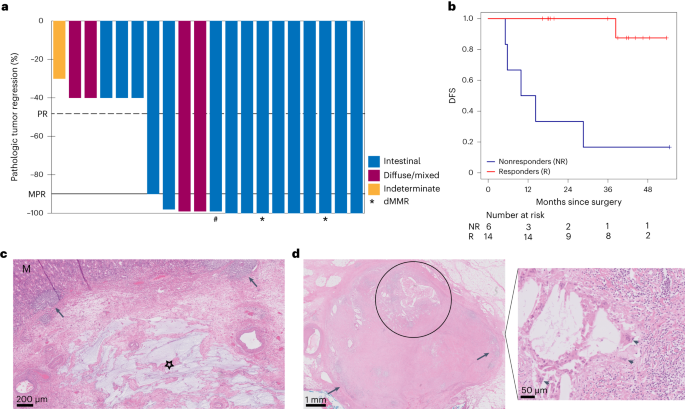
The phase 2 PANDA trial investigated the safety, efficacy, and immunologic correlates of atezolizumab plus chemotherapy in patients with resectable, nonmetastatic gastric and gastroesophageal junction adenocarcinoma. The study found that the combination treatment led to high pathologic response rates, with 70% of patients showing major pathologic responses and 45% achieving pathologic complete responses. Pathologic response was associated with significantly higher disease-free and overall survival rates. Additionally, circulating tumor DNA status was associated with response and disease-free survival, and biomarker analyses suggested that the functional status of CD8+ T cells may be a critical parameter in predicting response to the treatment.
Reading Insights
0
0
77 min
vs 78 min read
99%
15,502 → 100 words
Want the full story? Read the original article
Read on Nature.com