FDA Panel Rejects Medtronic's Symplicity Spyral RDN
TL;DR Summary
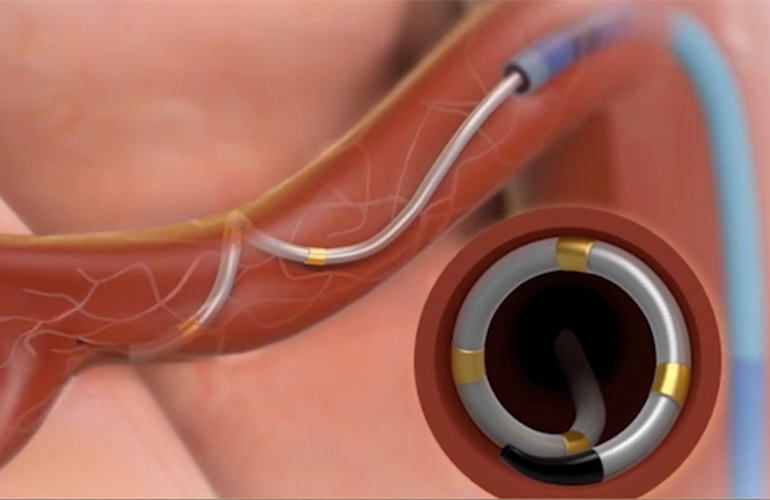
An FDA review panel has voted against recommending approval of Medtronic's Symplicity Spyral renal denervation (RDN) therapy for hypertension, stating that the risks outweigh the benefits. While the panel unanimously agreed that the catheter system is safe, they were divided on its efficacy. The panel chair cast the deciding vote against FDA approval. Medtronic's RDN trials have failed to meet the primary endpoint for efficacy, but the company remains hopeful and will work with the FDA to address the panel's concerns. The FDA review panel also supported the approval of a competing RDN technology developed by ReCor Medical.
FDA review panel votes against Medtronic's Symplicity Spyral RDN Medical Design & OutsourcingView Full Coverage on Google News
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
7 min
vs 8 min read
Condensed
94%
1,574 → 98 words
Want the full story? Read the original article
Read on Medical Design & Outsourcing