FDA Updates COVID Vaccine Guidelines and Access
TL;DR Summary
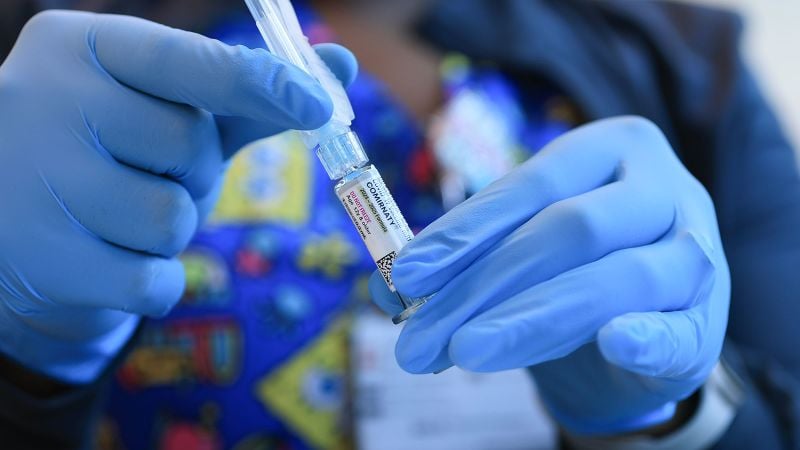
The FDA has narrowed Covid-19 vaccine approval to high-risk groups, but officials claim vaccines remain available to all. Experts warn that for healthy individuals under 65, access may be limited due to the need for off-label prescriptions, insurance issues, and logistical barriers, making true universal access unlikely. The situation is complicated further by state-specific regulations and the rescinding of emergency authorizations for certain age groups, raising concerns about equitable vaccine availability amid ongoing Covid-19 risks.
Topics:business#covid-19#fda-approval#health#healthcare-barriers#off-label-prescribing#vaccine-access
- After narrowing Covid-19 vaccine approval, the FDA says healthy people can still get it. But access might be complicated CNN
- Can You Still Get the Covid Shot? The New York Times
- New FDA coronavirus vaccine rules bring U.S. closer to other countries The Washington Post
- FDA approves fall Covid shots, but with new restrictions NBC News
- COVID vaccine guidance has changed — again. A doctor tackles your questions NPR
Reading Insights
Total Reads
0
Unique Readers
7
Time Saved
4 min
vs 5 min read
Condensed
92%
932 → 75 words
Want the full story? Read the original article
Read on CNN