FDA to review Moderna’s mRNA flu vaccine after new study plan
TL;DR Summary
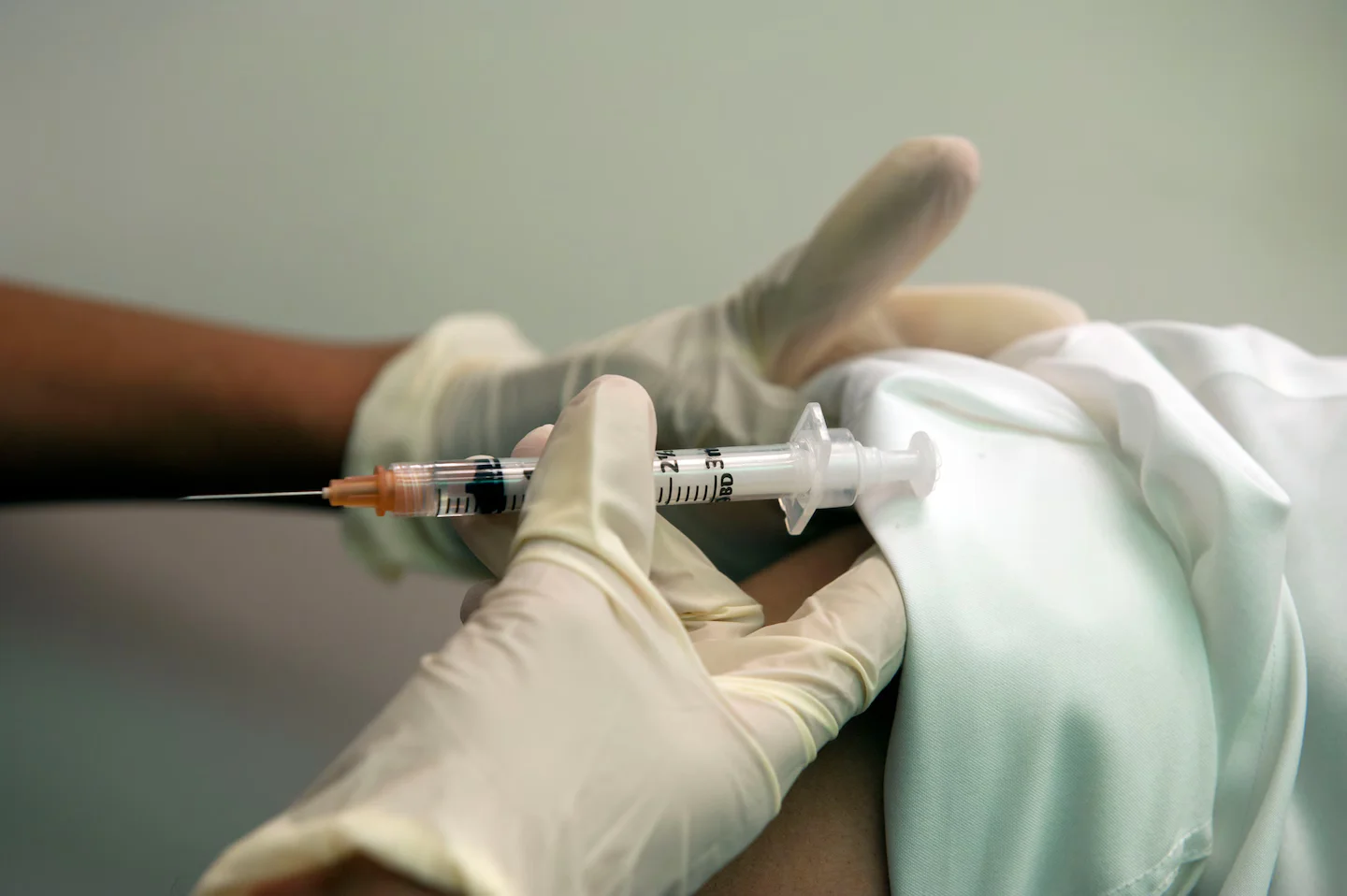
The FDA reversed its earlier decision and will review Moderna’s first mRNA-based influenza vaccine after Moderna agreed to conduct additional studies in older adults under a revised review process.
- FDA reverses course and will review Moderna’s mRNA-based flu shot The Washington Post
- F.D.A. Reverses Decision and Agrees to Review Moderna’s Flu Vaccine The New York Times
- FDA's reversal on Moderna flu shot bid followed White House pressure Politico
- U.S. rejection of new mRNA flu vaccine 'sends chills,' epidemiologist says PBS
- FDA agrees to review Moderna’s mRNA flu vaccine application in a reversal CNBC
Reading Insights
Total Reads
0
Unique Readers
2
Time Saved
18 min
vs 19 min read
Condensed
99%
3,633 → 29 words
Want the full story? Read the original article
Read on The Washington Post