FDA Recalls Over 580,000 Blood Pressure Bottles Due to Carcinogen
TL;DR Summary
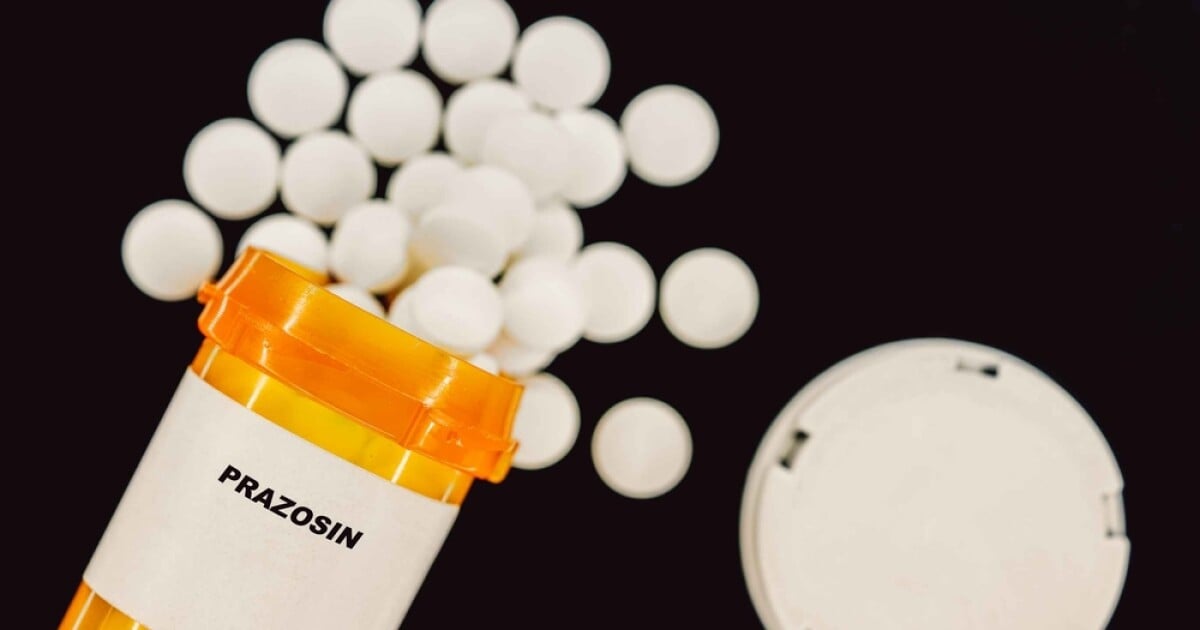
Over half a million bottles of Teva's blood pressure drug prazosin hydrochloride have been recalled due to elevated levels of a carcinogenic impurity, with the FDA classifying the recall as a Class II risk, indicating potential temporary health effects.
- Half a million bottles of blood pressure drug recalled for carcinogen levels Scripps News
- Blood pressure medicine recalled over cancer-causing chemicals above safety limits kare11.com
- Blood pressure medication recalled over high levels of cancer-causing chemical NBC 5 Chicago
- FDA recalls blood pressure medicine over cancerous chemical USA Today
- FDA Announces Nationwide Recall of Over 580,000 Bottles of Blood Pressure Medication Due to Cancer-Causing Chemical Health: Trusted and Empathetic Health and Wellness Information
Reading Insights
Total Reads
0
Unique Readers
8
Time Saved
1 min
vs 1 min read
Condensed
79%
188 → 39 words
Want the full story? Read the original article
Read on Scripps News