FDA Implements New Restrictions on COVID-19 Vaccine Eligibility and Approval
TL;DR Summary
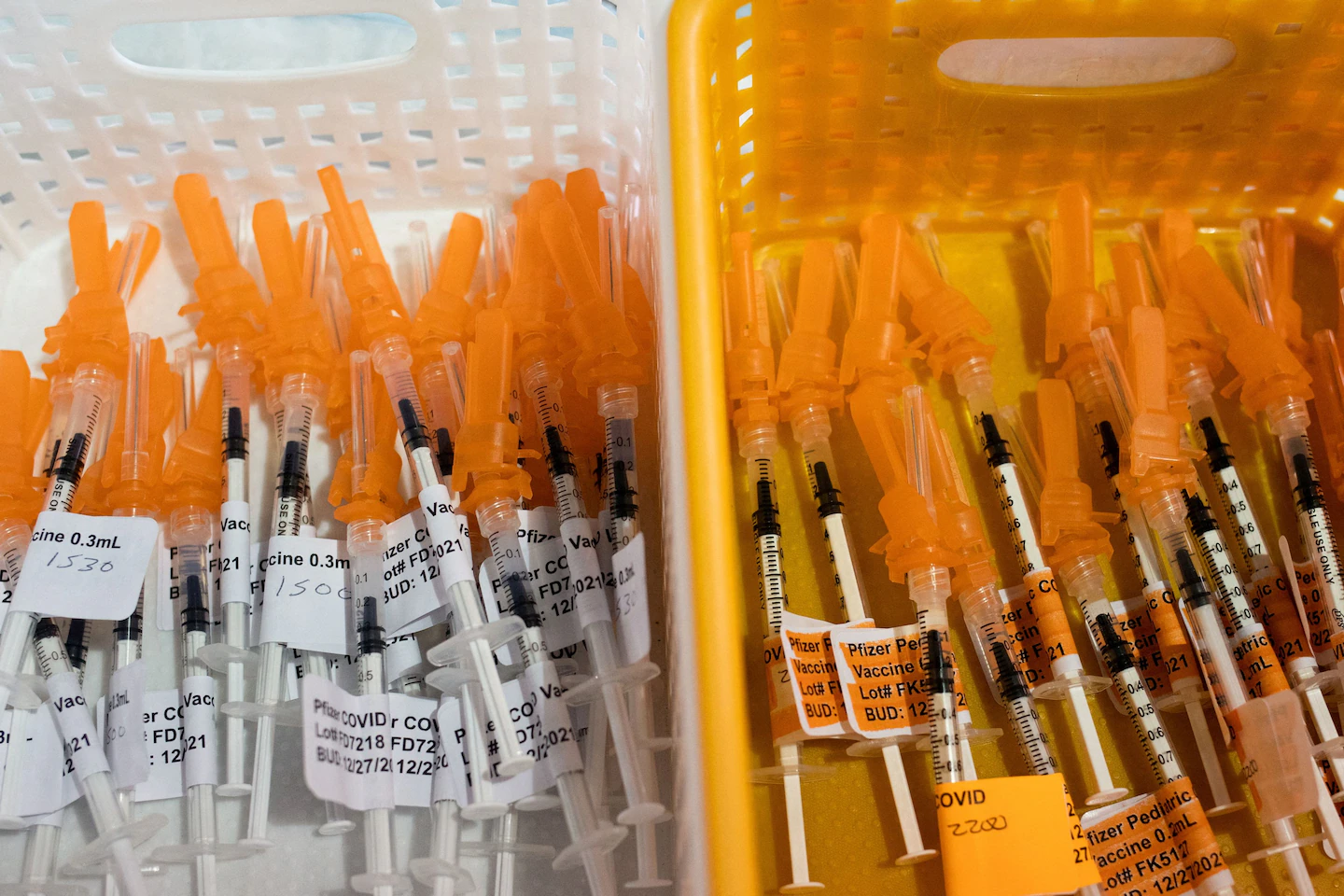
The FDA has limited approval for coronavirus vaccines to high-risk individuals, approving Pfizer, Moderna, and Novavax vaccines for this group amid a summer wave of cases, as companies work to update their formulations.
- FDA limits approval for new coronavirus vaccines to high risk people The Washington Post
- F.D.A. Approves Covid Shots With New Restrictions The New York Times
- RFK Jr. limits who is eligible for COVID shots Axios
- The latest COVID vaccines come with new FDA limits NPR
- Pfizer and BioNTech’s COMIRNATY® Receives U.S. FDA Approval for Adults 65 and Older and Individuals Ages 5 through 64 at Increased Risk for Severe COVID-19 Business Wire
Reading Insights
Total Reads
0
Unique Readers
1
Time Saved
0 min
vs 1 min read
Condensed
51%
68 → 33 words
Want the full story? Read the original article
Read on The Washington Post